You’ve Always Thought of Carbon as Just a Building Block — But the Bohr Model Brings Its Structure to Life
You’ve Always Thought of Carbon as Just a Building Block — But the Bohr Model Brings Its Structure to Life
Carbon, the cornerstone of organic chemistry and life itself, continues to captivate scientists with its intricate atomic architecture. Thanks to the Bohr Model’s enduring framework, we now visualize carbon not merely as a symbol on the periodic table, but as a carefully arranged ensemble of protons, neutrons, and electrons—each orbiting in precise energy levels governed by quantum principles. This elegant representation reveals not just the number of electrons and neutrons, but illuminates why carbon’s bonding behavior is both exceptional and foundational to biochemistry.
From graphene’s strength to DNA’s complexity, the Bohr Model underpins our understanding of carbon’s unique electron configuration and its pivotal role across science and technology.
Shells and Subshells: Mapping Carbon’s Electron Arrangement
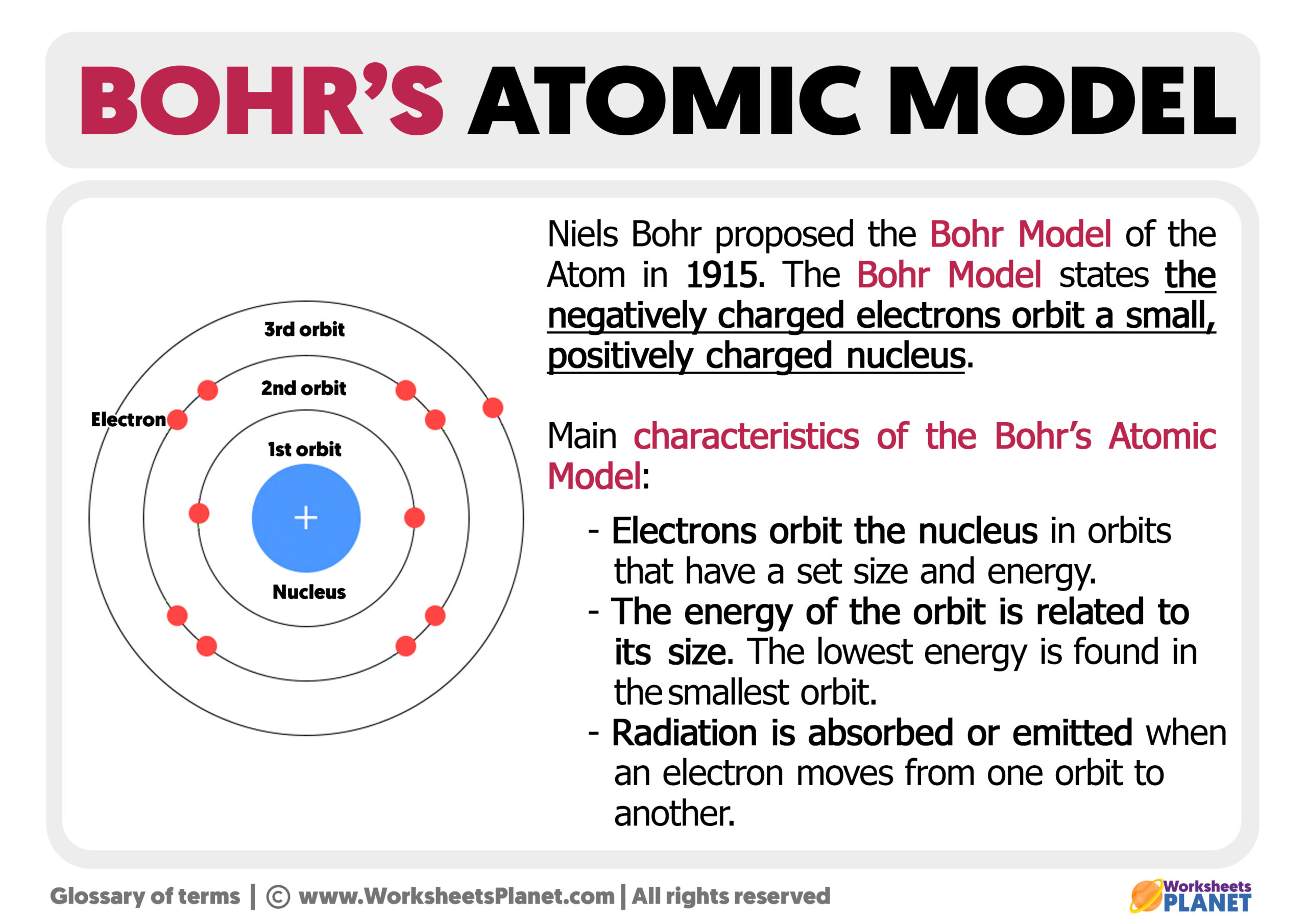
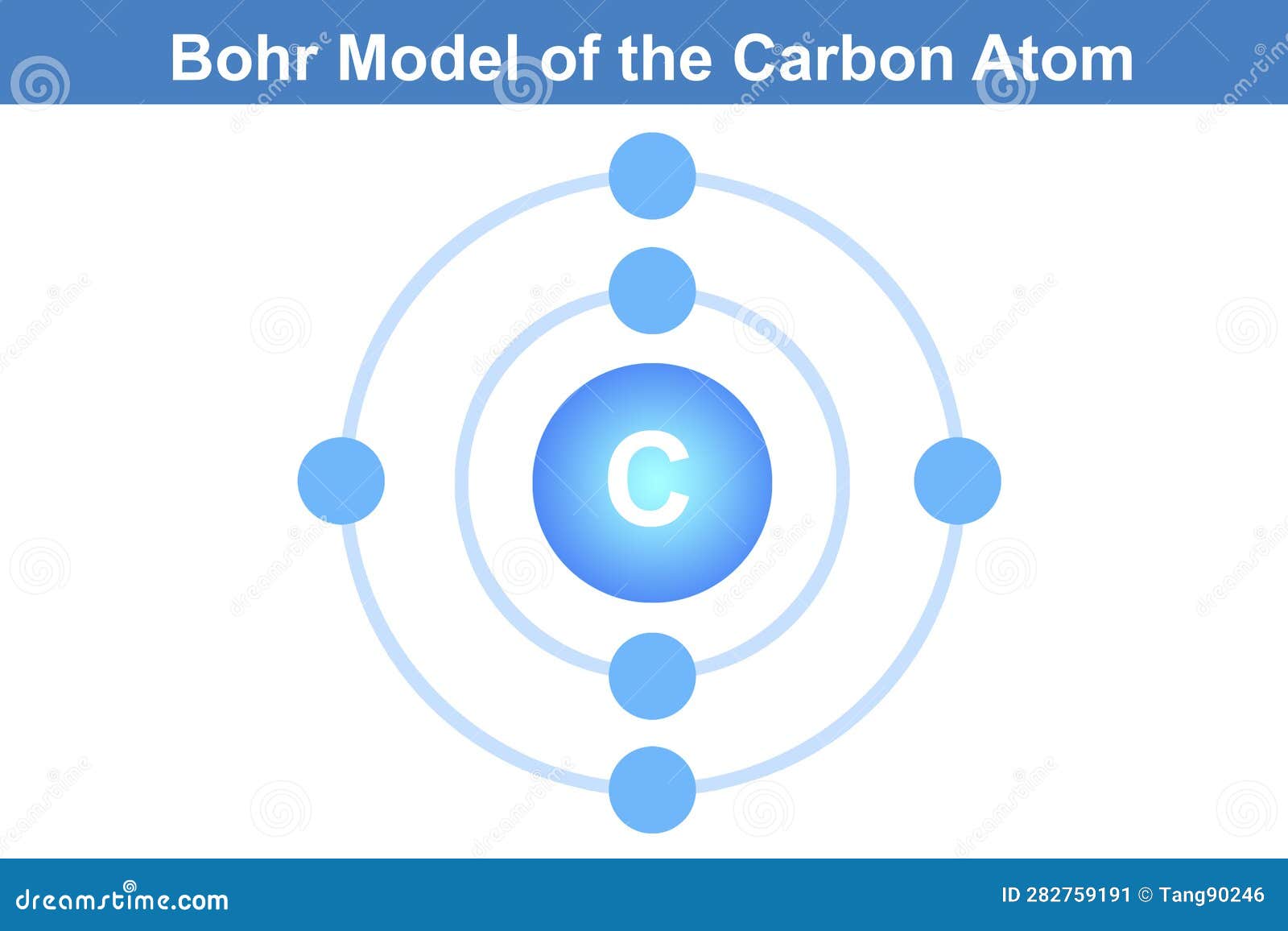
According to the Bohr Model—refined through quantum mechanics—carbon’s electrons occupy discrete energy shells around the nucleus, with specific capacities defined by the formula 2n², where n is the shell number. For carbon (atomic number 6), the electron configuration is 1s² 2s² 2p². This means: - Two electrons fill the innermost 1s orbital - Two more occupy the 2s orbital, completing a stable outer shell configuration - The remaining two electrons distribute into the two 2p orbitals, adhering to Hund’s rule (maximum parallel spin before pairing)—a principle rooted in quantum orbital structure.
These energy levels—called electron shells—are not just abstract constructs; they directly dictate carbon’s capacity for covalent bonding. The model explains why carbon, with four valence electrons, excels at forming four stable covalent bonds, a trait unmatched by most elements. As physicist and chemist Richard Feynman noted, “Nature uses the Schrödinger equation to build reality, and the Bohr Model captures a visible fraction of that.” The alternation between energy levels and orbital filling creates the foundation for carbon’s versatility.
Energy Levels and the Quantum Logic Behind Carbon’s Bonds
The Bohr Model’s quantized energy levels clarify why carbon forms selective, high-strength bonds. The 2s and 2p orbitals store electrons that are neither too tightly held nor completely free—enabling them to share electrons without instability. When carbon bonds with hydrogen (C–H), the 2p electrons interact with hydrogen’s 1s electrons, forming a molecular orbital where electrons are delocalized across atoms.
In crystalline networks like graphite or diamond, each carbon atom shares electrons across extended lattices, producing unparalleled hardness or conductivity. This electron mobility, governed by quantum rules, is visually represented by the layered shell model derived from Bohr’s early insights.
The double bond in carbon dioxide (CO₂), where carbon shares one electron with each oxygen via pi orbitals, also traces back to the model’s framework: unpaired p-valence electrons form covalent bridges across energy-splitted orbitals. The model does not describe electron wavefunctions in full detail, but it simplifies quantum behavior into observable patterns—allowing chemists to predict reactivity, stability, and geometric preference with remarkable accuracy.
Carbon’s Diverse Allotropes Explained Through Electron Configuration
The Bohr Model, though classical, remains instrumental in explaining carbon’s remarkable allotropes: diamond, graphite, graphene, and fullerenes.
Each form arises from distinct orbital arrangements and bonding geometries dictated by electron occupancy. - **Diamond**: Every carbon is tetrahedrally bonded via sp³ hybrid orbitals, creating an infinite 3D network with unmatched rigidity. - **Graphite**: Sp² hybridization forms a layered sheet with delocalized 2p electrons—explaining conductivity and softness within planes, yet weak ties between sheets.
- **Graphene**: A single hexagonal layer retains sp² bonding, enabling electron movement like liquid, with applications in flexible electronics. - **Fullerenes**: Curved sp² systems form closed cages, where triggering the encapsulated electrons triggers concerted quantum reactions. These forms demonstrate how subtle shifts in electron distribution—mapped via quantum logic—generate macroscopic differences in hardness, electrical behavior, and reactivity.
The Bohr Model serves as a gateway to interpreting these phenomena, even as modern theory extends deeper into wave mechanics.
The Bohr Legacy in Modern Carbon Science
Though developed nearly a century ago, the Bohr Model endures as a vital educational and analytical tool in carbon research. It provides an intuitive scaffold for visualizing electron dynamics—critical in developing advanced materials, pharmaceuticals, and nanotechnologies.
Consider graphene: engineered at the atomic level to exploit carbon’s outermost p-electrons, its conductivity and strength derive from the very orbital hybridization the Bohr Model simplifies. Similarly, drug-receptor binding hinges on electron sharing patterns rooted in quantum shell theories. Contemporary computational chemistry relies on ab initio and density functional theories, which build upon Bohr’s foundational ideas—quantization still guides how electrons interact and stabilize complex molecular geometries.
As Professor Michael Phillips of MIT observed: “We don’t teach chemistry by ignoring Bohr; we use his model to make the quantum invisible, tangible.” The Bohr Model’s simplicity lets scientists preview bonding behaviors before diving into complex equations.
Understanding carbon through the Bohr lens bridges theory and application. It reveals not only why carbon bonds but how it shapes everything from life’s molecular machinery to cutting-edge carbon capture technologies.
This model isn’t a mere artifact—it’s a living framework steadily informing innovation.
Carbon’s story, told in orbiting electrons and energy levels, continues—invisible yet indispensable. From the subatomic dance of protons and electrons to the engineered marvels of modern material science, the Bohr Model remains a key to unlocking carbon’s enduring legacy.


Related Post
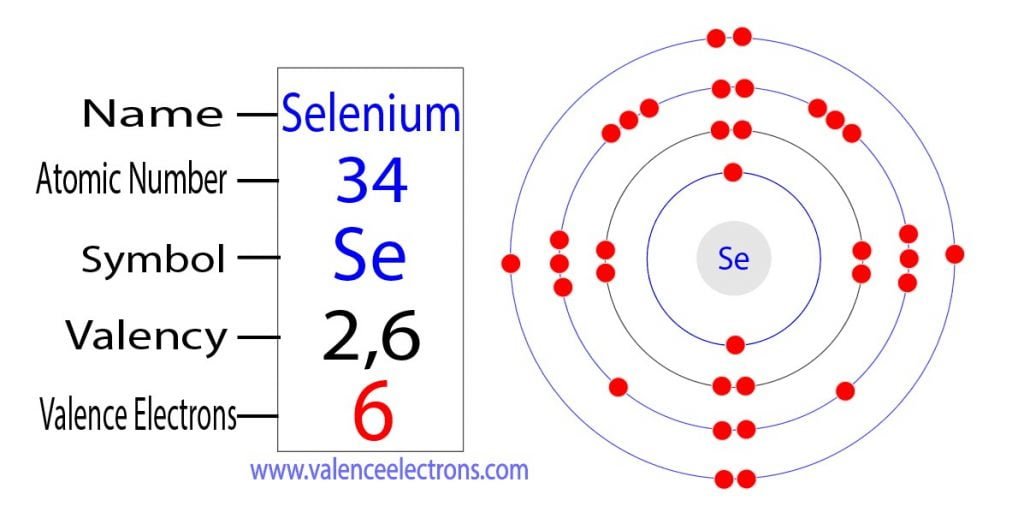
Se Valence Electrons: The Atomic Switches That Govern Chemical Behavior

The Fonz’s Enduring Legacy: Style, Substance, and Society’s Favorite Dilemma King

Campaign For Working Families: Empowering Jobs That Strengthen Communities

Gloria Borger’s Illness: A Journalist’s Battle and the Quiet Truth About Chronic Fatigue in the Media Elite

