X-Linked Recessive Inheritance Unveiled: How X-Linked Recessive Pedigree Charts Decode Inherited Rare Disorders
X-Linked Recessive Inheritance Unveiled: How X-Linked Recessive Pedigree Charts Decode Inherited Rare Disorders
In the intricate landscape of genetic inheritance, X-linked recessive conditions present a unique and often misunderstood pattern of transmission—one best understood through the precise use of X-linked recessive pedigree charts. These visual tools transform complex biological mechanisms into interpretable family histories, empowering clinicians, genetic counselors, and researchers to predict risks, guide testing, and illuminate the invisible threads of inherited disease. With leprechaun syndrome, color blindness, and hemophilia serving as textbook examples, understanding how X-linked recessive traits manifest across generations reveals far more than simple inheritance rules—it unlocks critical insights into genetic counseling, public health planning, and the future of precision medicine.
At the heart of X-linked recessive inheritance lies a fundamental principle of sex chromosome biology. Males, possessing only one X chromosome (XY), are far more frequently affected than females (XX), who require two copies of the defective gene—one on each X—to express the trait, a rare genetic coincidence. As Dr.
Sarah Lin, a clinical geneticist specializing in neurogenetic disorders, explains, “Because females can carry a functional X as a safeguard, the condition often ‘skips’ across generations, hiding in maternal relatives before emerging in sons.” This dynamic is vividly captured in X-linked recessive pedigree charts, which map familial relationships and allele transmission across multiple generations with surgical clarity. Pedigree analysis is not merely academic—it is the lens through which X-linked recessive disorders are diagnosed, tracked, and ultimately managed. A classic example lies in color blindness, the most prevalent X-linked recessive trait, affecting roughly 8% of males.
Pedigree charts reveal a descending pattern: a carrier mother (XᴺXᵘ) rarely shows symptoms, but her affected son (XᵘY) passes the trait to half her sons and none of her daughters. In contrast, rarer conditions like hemophilia A—a life-threatening clotting disorder—follow the same penetrant logic but present with severe clinical consequences that underscore the urgency of accurate pedigree mapping.
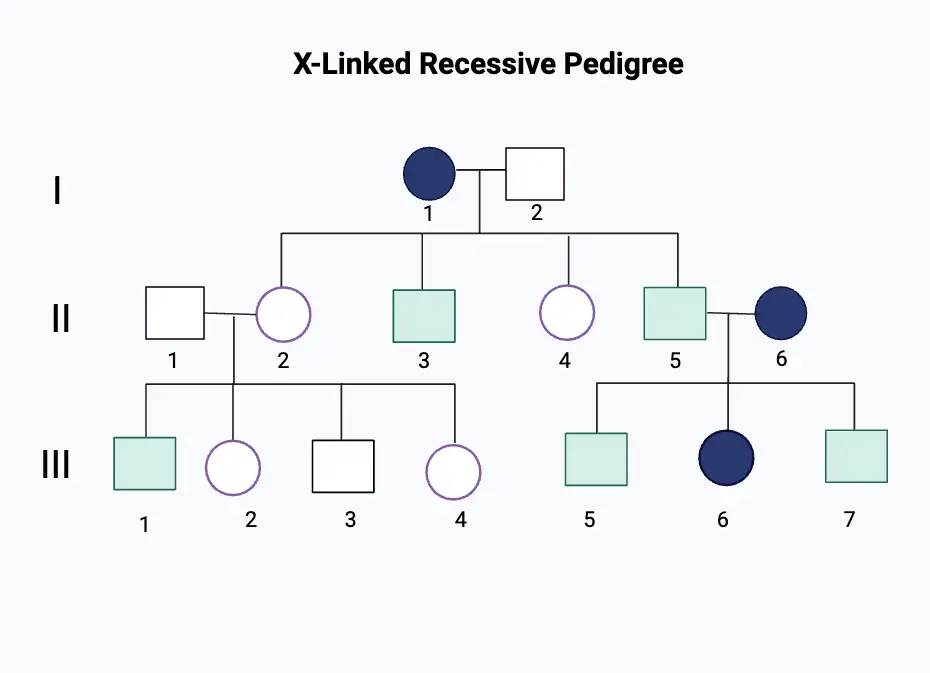
Building and interpreting X-linked recessive pedigrees follows a set of standardized conventions designed to minimize ambiguity.
Using Roman numerals for individuals (I, II, III…), symbols distinguish gender: open squares denote affected males, closed ones affected females, shaded shapes indicate carriers (heterozygous females), and half-shaded shapes reflect uncertain status. Importantly, unaffected females appear as closed squares to avoid misclassification—a detail vital for accurate inference. Generations are labeled horizontally, with vertical descent tracked rigorously.
This logical framework allows researchers and clinicians to trace inheritance across dozens of family members without confusion. Consider the well-documented pedigree of Duchenne muscular dystrophy (DMD), a catastrophic X-linked recessive disorder primarily fatal in childhood. In a typical family chart, an unaffected mother (XᴺXᵤ) may pass the DMD mutation (on her Xᵤ chromosome) to sons, who express the disease due to lack of a second functional X.
Daughters, receiving only her Xᵤ, become carrier females, often with mild symptoms or lifelong health impacts. Pedigree analysis reveals this “skipped” transmission across siblings and grandparents, exposing at-risk lineages before clinical onset. As genetic counselor Maria Chen notes, “These charts aren’t just static diagrams—they’re dynamic blueprints of risk, saving families years of diagnostic uncertainty.”
The symbolic language of X-linked recessive pedigrees encodes far more than simple transmission.
The absence of male-to-male passing—since affected sons cannot transmit the defect to father to son—is a defining feature, visible only in meticulously constructed charts. Females who inherit two copies (rare, as explained) appear shaded fully, while carriers (half-risk) display half-shading, reflecting their uncertain phenotype. Sibling clusters often reveal “jumping” generations, a telltale sign of recombination events that silence protective alleles and expose pathogenic variants.
These visual patterns transform abstract genetics into actionable familial insight, enabling early genetic testing and preemptive care. Pedigrees also highlight striking sex biases inherent in X-linked patterns. Males dominate affected cases not by greater risk, but by genetic vulnerability—each male has only one X chromosome, making expression of recessive alleles inevitable.
Females, with two Xs, remain largely phenotypically silent carriers unless mosaicism or second hits occur. This one-sided transmission sets X-linked recessive conditions apart from autosomal recessive or dominant patterns, reinforcing the necessity of gendered analysis in genetic assessment. During prenatal counseling, for instance, pedigree charts help estimate recurrence risks with precision—critical for families with a history of conditions like Hunter syndrome, where the likelihood of affected male offspring can exceed 50% based on maternal carrier status.
Beyond clinical genetics, X-linked recessive pedigree charts inform public health strategy and research priorities. Rare disorders, though individually uncommon, collectively affect millions, and understanding their inheritance accelerates targeted screening and resource allocation. Newborn screening programs for hemophilia now incorporate pedigree risk stratification, allowing early factor replacement therapy to mitigate long-term disability.
In rare disease registries, pedigrees enable longitudinal tracking of mutation prevalence and clinical progression across generations, feeding into genetic databases that fuel breakthrough drug development. Europe’s pioneering work on X-linked immunodeficiencies exemplifies this synergy. Collecting pedigrees from hundreds of families affected by Bruton tyrosine kinase deficiency, researchers identified founder mutations and traced inheritance through multi-generational lines.
This data was pivotal in designing gene therapy protocols now advancing through clinical trials—proof that X-linked recessive pedigrees are not merely descriptive tools but catalysts for therapeutic innovation.
The X-linked recessive pedigree chart stands as a masterful convergence of biology and visualization—a diagnostic compass in an age of genomic complexity. By mapping transmission across sex chromosomes with precision, these charts empower informed decision-making, reduce diagnostic delays, and strengthen family support systems.
As genetic medicine evolves, so too does the role of pedigrees: no longer static records, but dynamic, predictive tools shaping how rare genetic disorders are understood, managed, and ultimately conquered. In the hands of skilled practitioners, they remain indispensable—bridging the invisible science of inheritance to real-world health outcomes, one generation at a time. X-linked recessive inheritance, far from a theoretical curiosity, is a well-developed visual science that transforms genetic risk into actionable knowledge.
Through rigorous pedigree construction and interpretation, clinicians decode the silent patterns of X-chromosome-linked disease, offering families clarity, guidance, and hope. In this visual language of inheritance, every square, shade, and generation tells a story—one of resilience, risk, and the power of understanding.



Related Post
Inside Ground Zero Map Tarkov: The Battleground Where War and Reality Collide

Annie Thomas Miss India: Redefining Beauty, Resilience, and Representation

Downloader.Ia: The Next Evolution in Content Acquisition Technology

Emily Compagno’s Path to New Husband: A Modern Reimagining of Marriage and Love

