What Is Molecular Solvation?
What Is Molecular Solvation?
Molecular solvation—the dynamic interaction between solute molecules and surrounding solvent particles—is a foundational concept in chemistry and biology, governing everything from drug dissolution to enzyme function. At its core, solvation describes the process by which solvent molecules arrange themselves around a dissolved solute, stabilizing it through a network of electrostatic and dynamic forces. “Solvation is not just dissolution,” explains chemist Dr.
Lena Liu, “it’s the precise rearrangement of solvent molecules that dictates how a substance behaves in solution.” This intricate dance shapes physical properties, chemical reactivity, and biological activity, making it indispensable to scientific understanding. From pharmaceutical development to environmental chemistry, molecular solvation underpins key phenomena that define modern science.
Defining Solvation at the Molecular Level
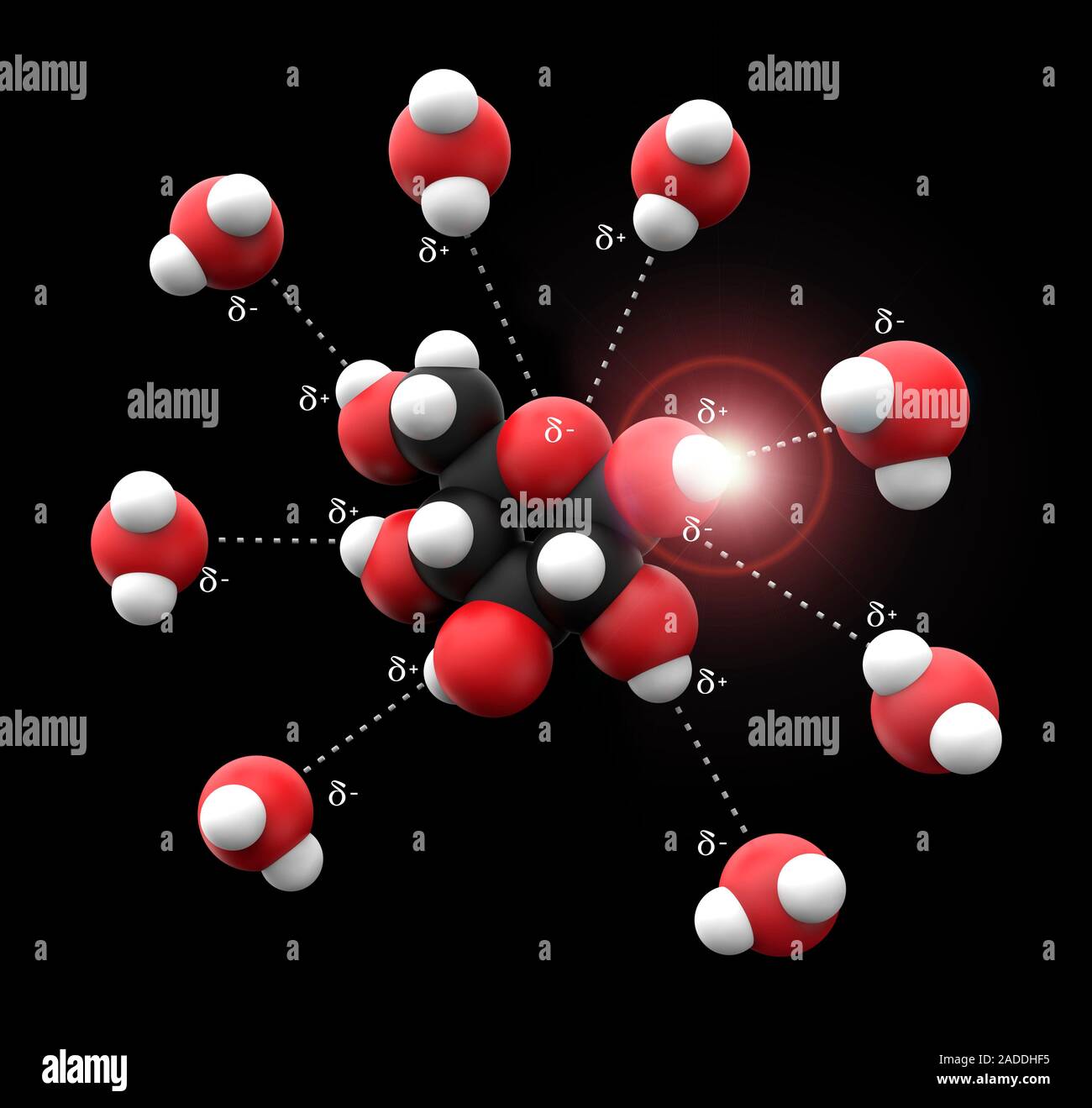
Molecular solvation occurs when a solute molecule—whether an ion, polar molecule, or organic compound—interacts with solvent molecules through non-covalent interactions including hydrogen bonding, dipole-dipole forces, and charge solvent effects.
Unlike mere mixing, solvation involves structured solvent reorganization: water molecules bending around solutes, oxygen and hydrogen atoms aligning near charged species, and solvent molecules forming transient cages that stabilize the solute. These interactions are not random—they follow thermodynamic principles, minimizing free energy to reach equilibrium. The strength and nature of solvation depend on the polarity and charge distribution of both solute and solvent.
Polar solvents like water solvent well substances with permanent dipoles, while nonpolar solvents prefer hydrocarbon solutes. This selectivity explains phenomena such as “like dissolves like,” where ethanol readily mixes with water but not with oil. “The architecture of solvation shells defines solute behavior in solution,” notes Dr.
Raj Patel, a physical chemist at MIT. “It’s a quantum-level negotiation of forces that determines solubility, reactivity, and stability.”
Mechanisms of Solvation: From Ions to Macromolecules
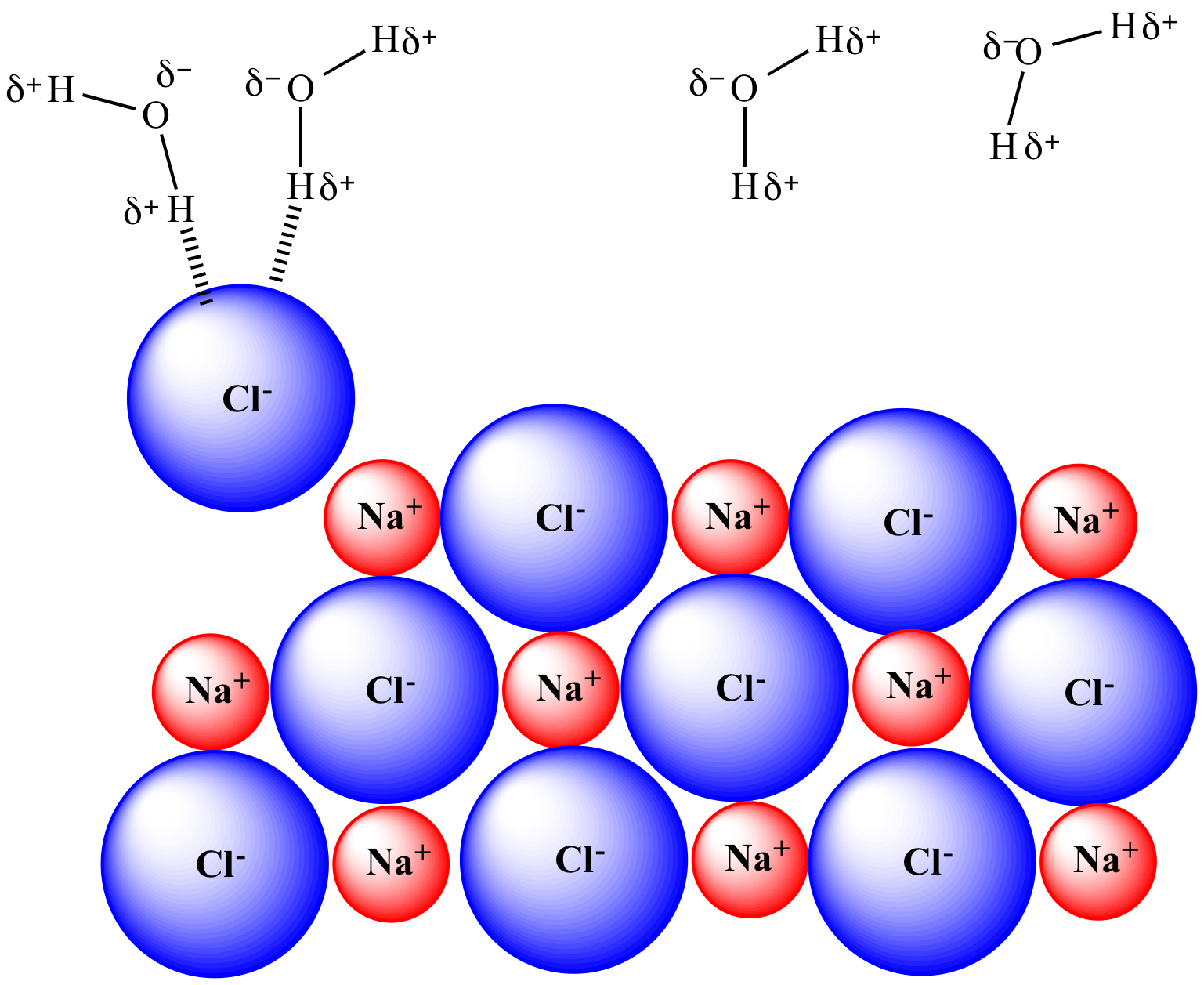
Solvation unfolds through distinct mechanisms tailored to the solute type. For ionic compounds, solvation involves ion-dipole interactions—charged solute ions surrounded by oppositely charged solvent molecules.
Sodium ions, for example, are deeply solvated by water’s oxygen atoms, a process critical to electrolyte function in biological systems. Meanwhile, polar molecules solvate via directional hydrogen bonding: ethanol’s hydroxyl group binds strongly with water, disrupting hydrogen networks and altering solvent viscosity. Macromolecular solutes like proteins experience more complex solvation.
Their extended structures induce rich, structured solvation layers that influence folding, stability, and function. “In proteins, solvation isn’t uniform; water molecules edge into cavities, hydrogen-bonding with polar side chains while avoiding buried hydrophobic cores,” explains biophysicist Dr. Elena Torres.
This selective solvation modulates conformational dynamics, making it essential for enzymatic activity and signal transduction.
Solvation Energy and Its Thermodynamic Significance
Central to solvation is the concept of solvation energy—the thermodynamic cost of transferring a solute from vacuum into solvent. This energy depends on the solute-solvent interaction strength, encapsulated in quantities like implicit or explicit solvent models used in computational chemistry.
A negative solvation energy indicates favorable dissolution; positive values suggest the solute is less stable in solution, predicting poor solubility. “The magnitude and sign of solvation energy determine phase behavior and reaction spontaneity,” clarifies Dr. Marcus Chen, a computational chemist.
“It’s the invisible engine driving dissolution, protein folding, and even battery electrolyte design.” Observing real-world applications, researchers use solvation energy to predict drug solubility, optimize industrial processes, and assess environmental contaminant mobility.
Applications Across Science and Industry
The impact of molecular solvation spans disciplines. In pharmaceuticals, understanding solvation guides drug formulation—ensuring active ingredients dissolve efficiently for optimal bioavailability.
Poor solvation often limits therapeutic success, driving innovations like nanoparticle encapsulation or surfactant addition to enhance aqueous stability. Environmental chemistry also hinges on solvation dynamics. When industrial solvents leak into soil or water, their solvation behavior dictates mobility, degradation rates, and ecological impact.
“How a pollutant interacts with water—or soil—dictates its fate,” says environmental chemist Dr. Annika Lund. “Solvation science helps identify risks and develop cleanup strategies.” In biochemistry, enzyme-substrate interactions depend on precise solvation layers that facilitate catalysis.
Moreover, industrial chemical reactions are optimized by manipulating solvent environments—visible in petroleum refining or polymer synthesis—where tailored solvation accelerates reaction paths and boosts yield.
Beyond the Basics: Solvation in Novel Materials and Emerging Research
Cutting-edge research explores solvation at nanoscales and in exotic solvents. In nanotechnology, solvation governs nanoparticle stabilization and aggregation, critical for drug delivery systems and catalytic nanoparticles.
“At the nanoscale, solvent structure near surfaces differs drastically,” explains Dr. Tariq Farooq, a materials scientist at Stanford. “Controlling solvation here unlocks new functionalities.” Supercritical fluids and ionic liquids—emerging solvent alternatives—challenge classical solvation models.
With tunable polarity and thermal behavior, these solvents enable green chemistry breakthroughs, from cleaner extraction to sustainable energy storage, where solvation principles remain central. “Solvation isn’t static—it evolves with solvent state,” observes chemist Dr. Nina Cho.
“Studying it across phases expands frontiers in synthesis and environmental remediation.”
The Silent Architect of Chemical Reality
Molecular solvation, though often invisible, is the silent architect of chemical behavior in solution. Far more than a passive backdrop, it actively shapes reactivity, stability, and interaction networks that underpin everyday phenomena and advanced technologies. From microscale enzyme catalysis to macroscale industrial processes, solvation defines how substances dissolve, react, and function.As scientific inquiry deepens, solvation science continues to reveal subtle mechanisms that govern life, matter, and innovation. Appreciating this process is essential for anyone seeking to master chemistry’s complex, dynamic reality.


Related Post
Indonesia Uncovered: Where History, Culture, and Adventure Collide
Teaching Coordination, Patience, and Sweet Math with Thorn and Balloons on Hooda Math
IPChicken: What Is It And How Does It Work?

Paris Taxi Strike Today: All You Need to Know From Labor Unrest to Daily Impact