What Is Atm in Chemistry? The Hidden Role of Atomic Mass in Molecular Behavior
What Is Atm in Chemistry? The Hidden Role of Atomic Mass in Molecular Behavior
The atomic mass, often abbreviated or referenced in scientific shorthand as ATM, plays a foundational yet underappreciated role in chemistry—serving not merely as a number in the periodic table, but as a critical determinant of molecular structure, reactivity, and physical properties. Often misunderstood as just a bulk property of elements, atomic mass profoundly influences how atoms interact, bond, and influence the behavior of everything from simple gases to complex organic compounds. Far more than a static label, atomic mass is a dynamic parameter embedded in the very mechanics of chemical systems.
At the heart of atomic mass lies the concept of atomic weight, defined as the weighted average of an element’s isotopes based on their natural abundance. Unlike the number of protons and neutrons in a specific isotope, atomic mass reflects statistical balance—each isotope contributes proportionally to the element’s overall identity. For instance, carbon에retsумا kann 있다.
碳的原子质量约为12.01 u(原子单位),反映了其所有稳定同位素(如碳-12、碳-13、碳-14)按自然分布所求的加权值。这种统计特性直接影响碳在生物大分子如蛋白质和DNA中的构建效率,以及在同位素分馏等精密化学过程中所表现出的行为差异。化学家在设计分子模拟或定量反应时,必须依赖精确的原子质量数据,以确保模型与实际相符。 atomic mass exert profound influence across multiple domains of chemistry. In physical chemistry, it determines gas density and pressure relationships, central to gas laws and kinetic theory—where Avogadro’s number and molar mass converge to link microscopic particle behavior with macroscopic observables like pressure and volume. As noted in *Physical Chemistry*, “...the ideal gas equation PV = nRT embeds molar mass intrinsically, linking atomic mass directly to volumetric response under fixed temperature and pressure.” This dependency reveals atomic mass not as a passive descriptor, but as an active variable shaping gas-phase dynamics.
In solution chemistry, atomic mass governs molecular weight—a key factor in colligative properties such as boiling point elevation and freezing point depression. These phenomena depend on the number of solute particles in a solvent, not their identity, meaning atomic mass becomes instrumental in predicting how solutes alter phase transitions. For example, adding a single sodium chloride ion raises the solution’s freezing point slightly, but multiplying that effect by thousands of equivalent moles—governed by atomic mass—predicts measurable, scientific outcomes.
Beyond bulk behavior, atomic mass shapes isotopic effects, where slight mass differences alter reaction rates. Kinetic isotope effects, particularly with hydrogen and deuterium, demonstrate that lighter isotopes react faster due to lower zero-point energy, a principle exploited in mechanistic studies and drug development. Here, atomic mass is not just a measurement—it is a functional parameter that guides chemical kinetics and design.
What Is Atm in Chemistry is thus


Related Post
[1478725862].jpg)
Jason Hoppy’s New Wife: Unflinching Look at Life After Divorce in the Spotlight
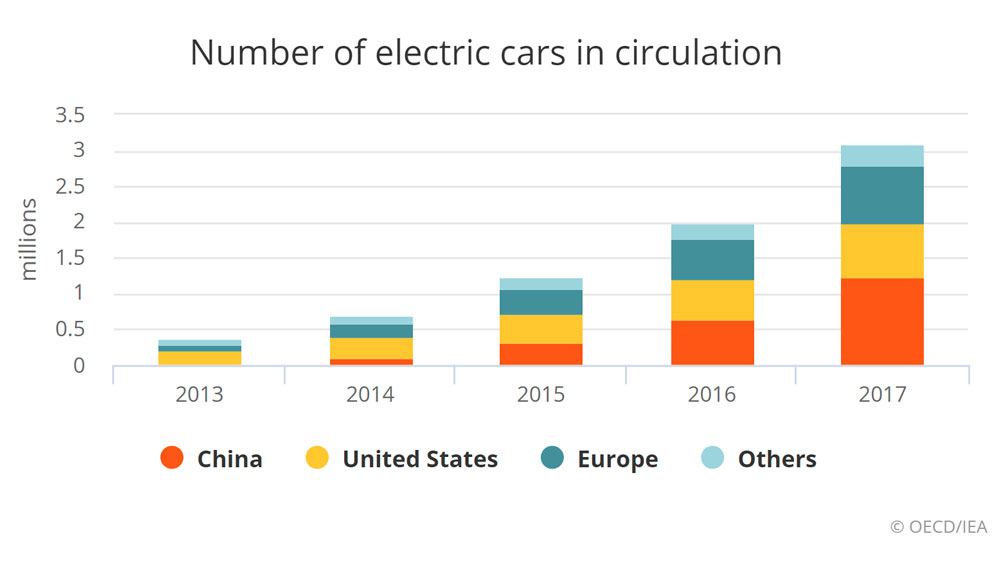
2021’s Unstoppable Rise: How Electric Vehicles Surpassed 10 Million Global Sales

Syleste Rodriguez Fox 10: Age, Marriage, and the Private Life Behind the Spotlight

Unveiling Lisa Niemi's Net Worth: A Comprehensive Look At Her Wealth

