What Is a Chemical Property? Unlocking the Invisible Forces That Define Substance Behavior
What Is a Chemical Property? Unlocking the Invisible Forces That Define Substance Behavior
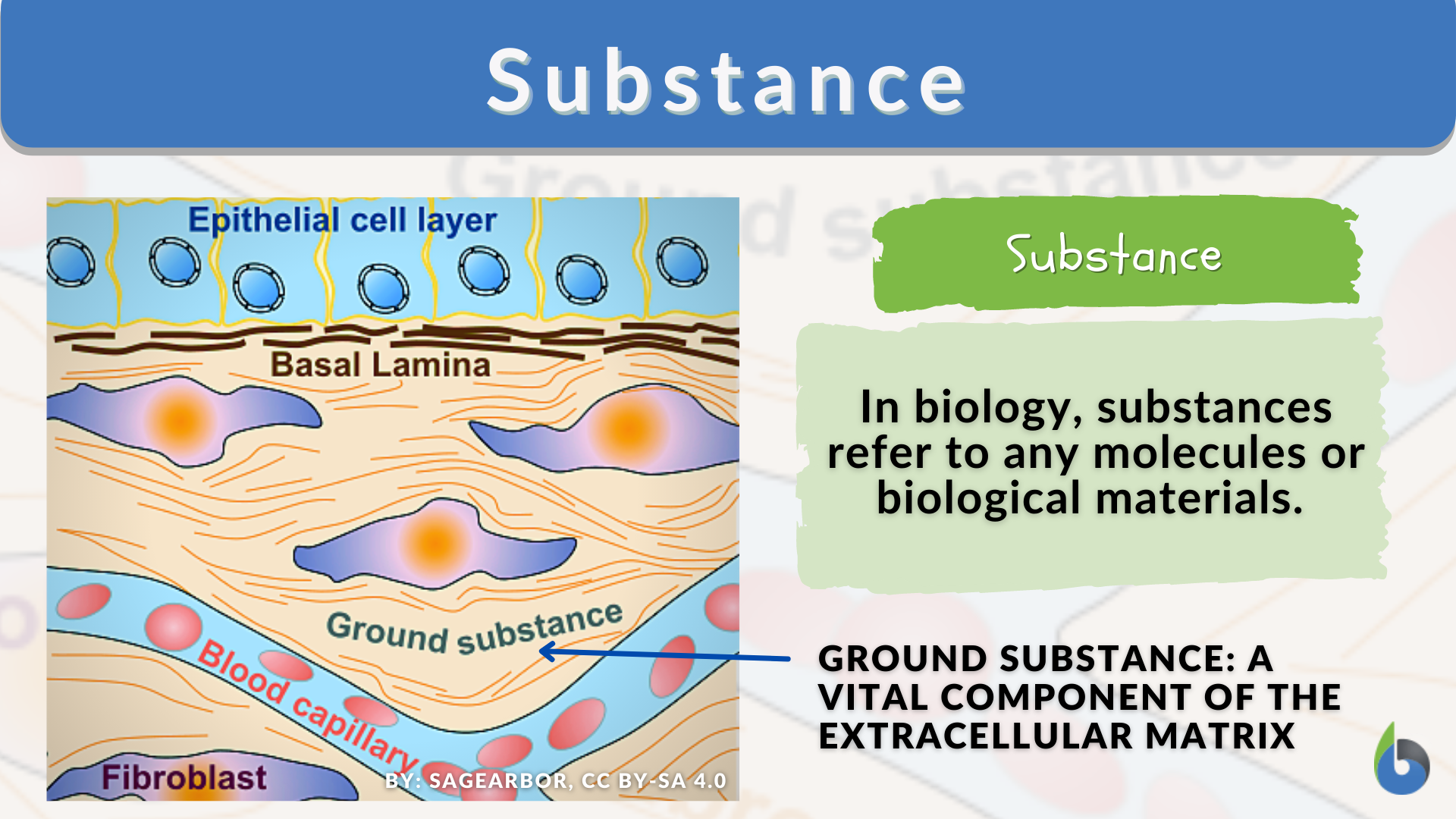
Every interaction between materials hinges on far more than appearances— beneath the surface lies a complex network of invisible forces governed by chemical properties. While physical properties describe observable traits like color, density, and melting point, chemical properties reveal what a substance can or cannot do during reactions: whether it burns, reacts, corrodes, or degrades. These intrinsic characteristics determine how materials behave in nature and industry, making them essential to chemistry, engineering, medicine, and environmental science.
In essence, chemical properties define a substance’s reactivity and transformation potential—what makes a material truly active at a molecular level.
For example, when iron rusts, it doesn’t just change surface color—it breaks down into iron oxide, a distinct new compound, releasing energy in the process. This shift reveals two key chemical properties: reactivity with oxygen and the ability to oxidize. These properties are rooted in the arrangement of electrons, molecular structure, and the strength of bonds within molecules.
When atoms gain or lose electrons—ionization—they reveal reactivity trends, such as alkali metals’ explosive reactivity or noble gases’ reluctance to react. Molecular structure further influences how substances interact: polymers resistant to hydrolysis maintain stability in water, showcasing chemical inertness.
Antibiotics like penicillin target bacterial enzymes through specific, reversible reactions, while carbamazepine, used for epilepsy, relies on its stability and metabolism in the liver. Designing drugs demands understanding how a compound reacts with biological molecules without premature breakdown. In energy storage, lithium-ion batteries depend on controlled oxidation-reduction reactions.
Lithium’s high reactivity with oxygen makes it ideal for anodes, where it releases electrons during discharge. “Controlling chemical properties—stability during charge, reactivity in redox flow—is what makes modern batteries efficient and safe,” says Dr. Amina Okoye, an electrochemist at a leading battery research lab.
Environmental science also hinges on chemical behavior. Acid rain forms when sulfur dioxide reacts with atmospheric moisture to produce sulfuric acid, damaging ecosystems and infrastructure. Conversely, activated carbon filters capture pollutants by adsorption, leveraging surface chemistry to remove impurities.
“Understanding these properties allows us to predict environmental impacts and engineer solutions,” explains Dr. Marcus Liu, a chemist specializing in remediation technologies. In manufacturing, chemical reactivity guides material selection.
Stainless steel’s chromium oxide layer prevents rust, keeping bridges and surgical tools corrosion-resistant. Polymers used in plastics undergo polymerization reactions that are carefully tuned: too reactive, and they degrade prematurely; too inert, and they won’t bond. These deliberate choices reflect deep chemistry behind engineered materials.



Related Post

How Deep Are the Sharks in Tiny Fishing? Uncovering Truths Beneath the Surface

The Untold Success Story: Kevin Gates’ Rise to Billionaire Status in 2024 – Forbes Serverumentary

How to Film POV While Flying iPhone Cameras: Mastering the Art at 30,000 Feet Amid Dubai’s Cockpit Ethics Clash
From Innovation to Delight: The Living Legacy of Hybrid Pastry Since 2013 and 2025’s Flavor Revolution

