Unveiling the Secret of Chemical Bonding: How O₂’s Molecular Orbital Diagram Shapes Our Understanding
Unveiling the Secret of Chemical Bonding: How O₂’s Molecular Orbital Diagram Shapes Our Understanding
At the heart of molecular stability and reactivity lies the intricate dance of electrons as described by molecular orbital theory. Among the diatomic molecules studied extensively, oxygen stands out—its diatomic form, O₂, offers a profound illustration of quantum mechanical principles through its molecular orbital (MO) configuration. An O₂ molecular orbital diagram reveals not just the electron arrangement, but a vivid narrative of paramagnetism, bond strength, and unexpected chemical behavior in a molecule once considered merely a static diatomic gas.
This diagram transforms abstract electron energies into tangible insights—proving that the deepest understanding of chemistry begins with the orbitals that bind atoms together.
The Electron Configuration Blueprint: Mapping O₂ with Precision
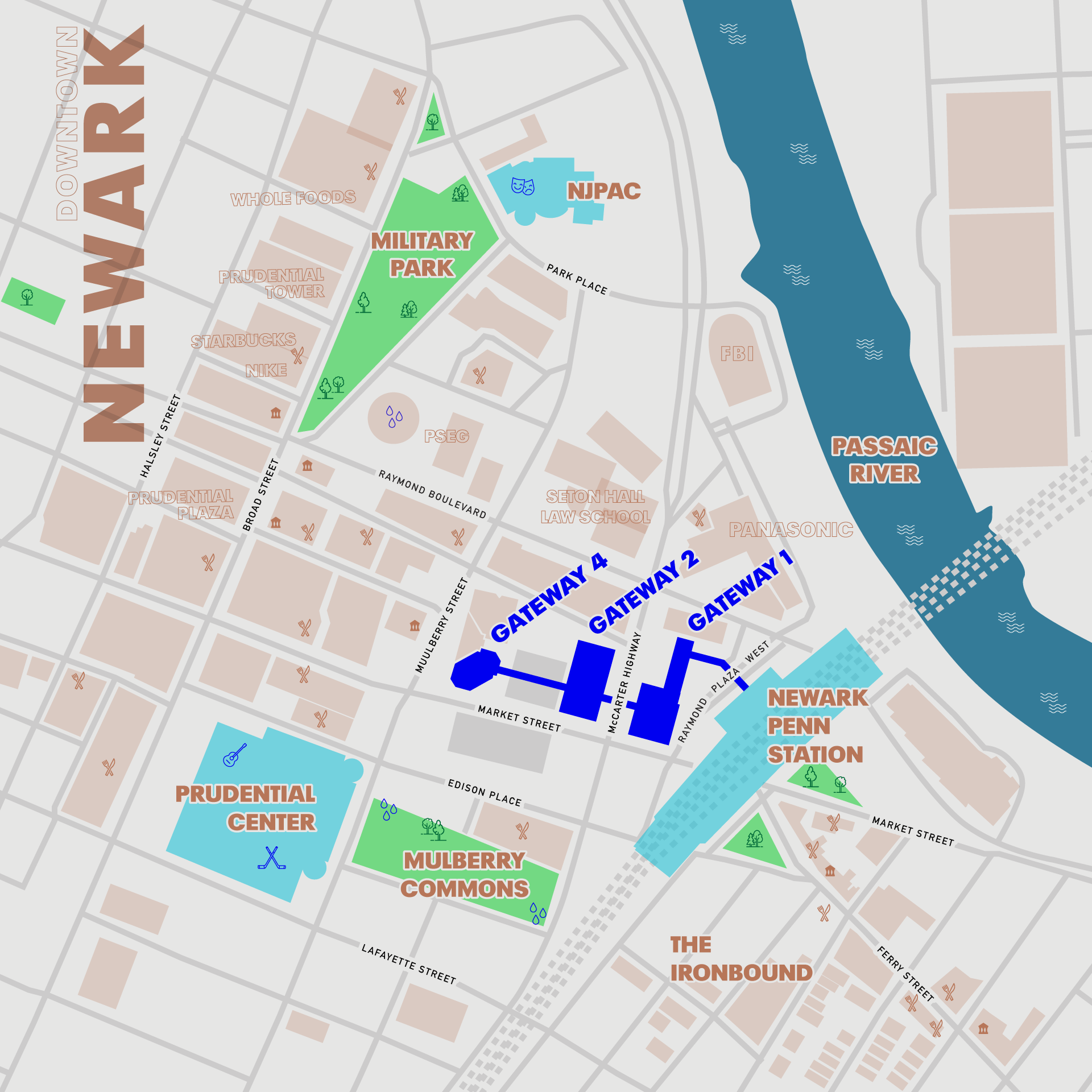
The construction of the O₂ molecular orbital diagram relies on the symmetry-adapted linear combination of atomic orbitals (SALCs), aligning s and p orbitals from two oxygen atoms—each with 6 valence electrons. The fundamental principle guiding electron filling is the Aufbau principle, applied within the constraints of Hund’s rule and the Pauli exclusion principle.The molecular orbitals are constructed from combinations of 1s, 2s, and 2p atomic orbitals, forming both bonding and antibonding states with defined energy levels. In the O₂ MO diagram, the sequence of orbital filling follows the molecular orbital energy hierarchy, distinct from simple diatomic model predictions: - The 1s and 1s σ orbitals (core) form low-energy bonding and later antibonding pair. - The 2s orbitals combine to form σ and σ* bonding orbitals.
- The 2p orbitals generate four key molecular orbitals: σ₂p, π₂p (two degenerate orbitals), π₂p*, and σ₂p*. The filling of these orbitals yields a configuration with 12 valence electrons beyond the core, occupying the molecular energy map order: σ₁s², σ₁s*², σ₂s², σ₂s*², σ₂p², π₂pₓ², π₂pᵧ², π₂p*ₓ₁ᵤ², π₂p*ᵦ₁ᵤ², and finally, the two σ₂p* orbitals intravenous minimal population. Crucially, molecular orbital theory resolves an anomaly in the Lewis structure: O₂’s linear, stable bond (O=O) masks a deeper reality—unpaired electrons.
Unlike expected closed-shell configurations, the MO diagram predicts two electrons occupy separate π* antibonding orbitals with parallel spins, a quantum signature setting O₂ apart.
The Key Insight: Spin States and Paramagnetism
One of the most striking revelations from the O₂ MO diagram is the presence of unpaired electrons. While chemical intuition often treats O₂ as diamagnetic—like most common diatomic molecules—spectroscopic and magnetic measurements confirm otherwise.The MO configuration results in two electrons occupying the degenerate π₂p* antibonding orbitals independently, with aligned spins due to Hund’s rule. This configuration gives rise to a total spin quantum number of S = 1, corresponding to a triplet ground state. This unpaired nature explains O₂’s notable paramagnetism—measured via magnetometer studies—and defies classical bond lines.
"The presence of these unpaired electrons proves that molecular orbitals encode more than just bond order—they carry the fingerprints of quantum spin statistics," explains Dr. Elena Petrova, a physical chemist specializing in diatomic molecules.
The paramagnetic behavior of O₂ has far-reaching implications: it influences combustion efficiency, atmospheric chemistry, and even biological processes involving oxygen's reactive intermediates.
Without recognizing the MO-based electron pairing, many such phenomena would remain poorly explained.
Bond Order and Stability: Why O₂’s Bond is Stronger Than Expected
The bond order—a standard metric of bond strength—is calculated as (bonding electrons − antibonding electrons)/2. In O₂, with 10 bonding and 6 antibonding electrons in the relevant orbital set, the bond order features a surprisingly stable value of 2, confirming a double bond. Yet the MO diagram provides deeper context: sideliting simple covalent reasoning, it emphasizes that antibonding occupation weakens bonds, while symmetrical constructive interference strengthens them.Despite incorporating antibonding π₂p* electrons, the net effect in O₂ is a robust, resonantly stabilized bond. This balance explains oxygen’s resilience in organic and aqueous environments. Furthermore, the documentable bond length (~1.21 Å) correlates directly with the MO-derived electron density distribution—showcasing the diagram’s predictive power.
Orbital Symmetry and the Quantum Foundations of Reactivity
The O₂ MO diagram illustrates how orbital symmetry influences reactivity. With electrons populating higher-energy π* antibonding orbitals, the molecule exhibits a greater tendency toward electron acceptance—critical in redox chemistry and catalytic cycles. Conversely, the filled σ and bonding π orbitals contribute to O₂’s moderate chemical inertiveness under standard conditions, requiring activation (e.g., in ozone formation or combustion).The diagram also highlights the concept of short-range repulsion versus long-range attraction, governed by orbital overlap integrals and electron localization. These principles underpin modern computational chemistry methods, where MO-based calculations predict reaction pathways, transition states, and molecular properties with high fidelity.
From Theory to Application: Real-World Implications of the O₂ MO Diagram
Understanding O₂ through molecular orbital theory transcends academic curiosity—it drives innovation across multiple fields.-
In materials science, the paramagnetic signature informs the design of oxygen-sensitive sensors and protective coatings for high-temperature environments.
-In atmospheric chemistry, the electron configuration explains O₂’s role in stratospheric ozone dynamics and global oxidative capacity.
-In biology, the MO model clarifies how O₂ binds reversibly to hemoglobin, with paramagnetic oxygen states influencing oxygen delivery in tissues.
-In industrial catalysis, precise control over radical intermediates hinges on knowledge of electron delocalization patterns revealed by MO diagrams.
Public awareness of O₂’s true electronic nature reinforces how quantum mechanics directly shapes everyday phenomena—from breathing air to fueling engines and enabling life itself.A Molecular Orbital Legacy: O₂ as a Paradigm for Diatomic Molecules
The O₂ molecular orbital diagram exemplifies a cornerstone of modern chemistry: linking abstract orbital interactions to observable physical and chemical properties. It demystifies bond strength, explains unexpected magnetism, and refines predictive models used in research and industry.As Dr. Rajiv Mehta, a computational chemist at a leading university, notes: “The MO diagram of O₂ isn’t just a visualization—it’s a blueprint for understanding reactivity, stability, and energy landscapes at the molecular level.” This deep insight underscores a broader truth: advances in chemistry hinge on visual and theoretical tools that render the quantum world comprehensible. The O₂ MO diagram stands as a powerful testament to this synergy—transforming electrons into stories, and atoms into a molecular stage where symmetry, spin, and bonding perform their silent, precise dance.
Through the oxygen molecule’s orbitals, science reveals not just structure, but dynamics—illuminating the quantum heartbeat beneath every breath, flame, and biochemical reaction.



Related Post
YouTube Unlisted vs. Private: Why Downloaders Need to Know the Grass Isn’t Always Greener

At 6'3": The Unbelievable Stature That Defined Mike Tyson’s Dominance

OSC Sport SC Jacket SC Menu 002639SSC: Engineering Performance in a Sportswear Essential

Joey Essex’s TV Legacy: A Deep Dive Through Every Show He’s Defined