Unlocking the Geometry and Chemistry of PCL₃: Decoding Its Lewis Structure and Molecular Behavior
Unlocking the Geometry and Chemistry of PCL₃: Decoding Its Lewis Structure and Molecular Behavior
Calcium pentachloride trifluoride (PCl₃) stands as a remarkable compound in inorganic chemistry, offering insights into molecular geometry, chemical reactivity, and industrial utility. At the heart of understanding PCl₃ lies its precise Lewis structure—a foundational tool that reveals electron distribution, bonding patterns, and the tetrahedral framework governing its physical and chemical properties. Through this structure, scientists decipher the compound’s polarity, reactivity, and anomalous behavior relative to phosphorus analogs.
This article explores the Lewis structure of PCl₃, its geometric implications, and the broader significance of its electron arrangement in both laboratory and industrial contexts.
The Lewis structure of PCl₃ begins with phosphorus, a group 15 element possessing five valence electrons, at the molecular core. Surrounded by three chlorine atoms—each with seven valence electrons—phosphorus forms three covalent bonds, utilizing three of its electrons, while distributing the remaining two as a lone pair.
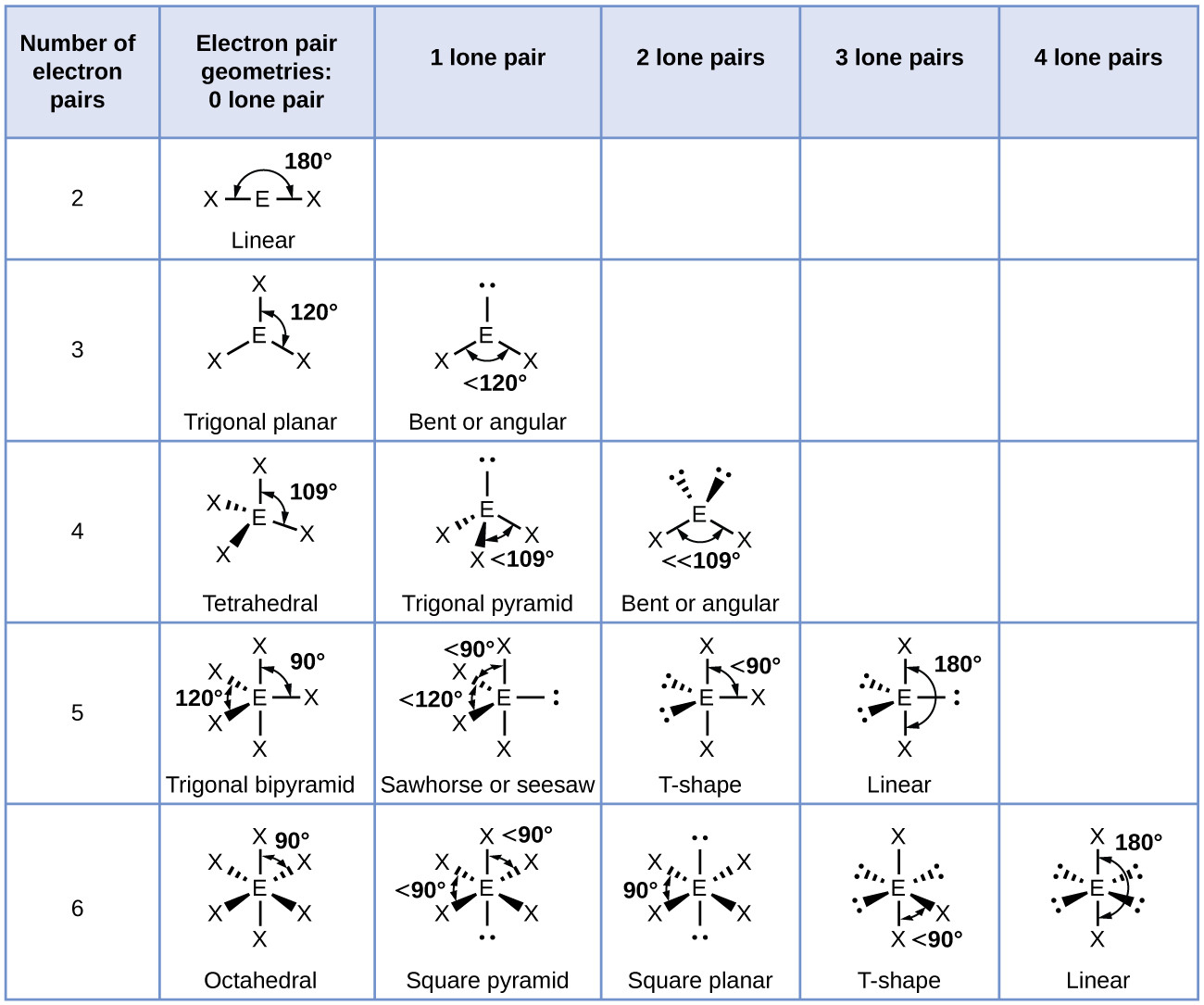
This configuration gives PCl₃ a trigonal pyramidal molecular geometry, a deviation from the ideal tetrahedral arrangement observed in molecules like CH₄ or PF₅. Unlike the symmetrical bond angles of a perfect tetrahedron, the presence of a lone electron pair compresses the P–Cl bond angles to approximately 100°, slightly less than the ideal 109.5°, introducing subtle electronic repulsion that defines the molecule’s shape.
At the core of its electron distribution, PCl₃’s Lewis structure reveals a dynamic interplay between bonding and non-bonding electrons. Phosphorus contributes one d-electron (part of its 3d orbitals in the ground state) to facilitate expansion of its valence shell beyond the octet, though PCl₃ itself adheres to a standard octet rule for phosphorus (Lewisan principle).
The three P–Cl single bonds Each contribute shared electron pairs; the lone pair occupies one sp³ hybrid orbital, reinforcing the molecule’s pyramidal form. This spatial asymmetry profoundly influences how PCl₃ interacts with other species, especially nucleophiles and electron-deficient compounds, enabling its role as a versatile reagent in fluorinating agents and solvent systems.
Geometric Influence: Why the Lone Pair Shapes PCl₃
The lone pair on phosphorus is the architect of PCl₃’s trigonal pyramidal configuration. Repulsion between the lone pair and bonding pairs distorts ideal tetrahedral angles, resulting in a distinct three-dimensional shape that impacts chemical behavior.In contrast to molecules with no lone pairs, such as ammonia (NH₃), which also adopts trigonal pyramidal geometry, PCl₃ differs due to the greater electronegativity and smaller atomic radius of chlorine. The stronger P–Cl bonds and chlorine’s ability to stabilize polarity amplify electrostatic effects, making the molecule more likely to participate in dipole-driven interactions. This structural nuance translates into measurable differences: PCl₃ exhibits moderate polarity, with a partial negative charge on chlorine atoms and a positive partial charge on phosphorus—drawn by electronegativity contrast.
While not as highly polar as, say, hydrochloric acid (HCl), this dipole moment enables solvation of ionic species and moderate compatibility with polar solvents, critical for its roles in organic synthesis and industrial chemical processing.
Chemical behavior is deeply informed by PCl₃’s electronic architecture. The lone pair serves as both an electron donor and a reactive site, making PCl₃ a potent electrophile and a reliable fluorinating agent in organic chemistry.
When reacting with alcohols or amines, the molecule readily accepts nucleophiles at phosphorus, generating fluoride salts and aminoplast derivatives—transformations central to pharmaceutical development and polymer chemistry.
Industrial and research applications underscore the importance of understanding PCl₃’s structure. Used in fluorination reactions, it enables controlled introduction of fluorine atoms into organic molecules—processes essential for synthesizing bioactive compounds and specialty materials. Its volatility and thermal stability also make it valuable as a solvent in high-temperature chemical transformations.
In battery and semiconductor research, PCl₃ derivatives influence ion transport due to their flexible coordination environments enabled by the lone pair-driven geometry.
Yet, PCl₃’s structure holds a subtle paradox: despite phosphorus’s capacity for expanded octets, PCl₃ remains a modest 3-coordinate species with a lone pair—explaining why it resists unusual oxidation states or distortions typical of hypervalent compounds. This stability, arising from the tight integration of bonding and lone pair electron density, underscores why PCl₃ resists extreme coordination changes, favoring consistent reactivity patterns in synthetic contexts.
Polyatomic Polarity and Environmental Considerations
PCl₃’s trigonal pyramidal geometry contributes to its net molecular dipole, a property increasingly scrutinized under environmental and safety regulations. Its polarity enhances solubility in polar media but raises concerns about aqueous persistence and potential bioaccumulation, common with chlorinated and phosphorus-based compounds. Understanding its structure allows chemists to predict degradation pathways and design safer handling protocols.Recent studies emphasize PCl₃’s role in sustainable synthesis, where its controlled reactivity—governed by its stereochemically defined geometry—enables selective transformations reducing waste. By tailoring ligands or reaction conditions that modulate phosphorus’ coordination environment, researchers aim to expand the compound’s utility while minimizing ecological footprint.
Ultimately, the Lewis structure of PCl₃ is far more than a schematic representation; it is a roadmap that reveals how electron pairing, bond angles, and molecular polarity converge to shape function.
From guiding synthetic strategy to informing industrial application, this simple yet profound geometry underpins PCl₃’s enduring relevance in chemistry. As research advances, the intricate dance of electrons within PCl₃’s trigonal pyramidal framework continues to inspire deeper understanding—illuminating pathways from the lab bench to the global chemical landscape.
In mastering PCl₃’s structural language, scientists not only decode a single molecule’s behavior but also refine tools for manipulating molecular interactions across disciplines, proving that even well-studied compounds hold keys to innovation.

Related Post
Legal Aid Evansville Indiana: Your Essential Guide to Affordable Justice

Apple News on Android: Your Guide to Accessing Apple’s Ecosystem Seamlessly

Discovering Listcrawler Louisville: The Ultimate Guide to Navigating the Bluegrass City Like a Local

Bob Kelly Fox 29: Age, Height, and Legacy of a Trailblazing Figure