Unlocking the Chemistry Behind Hydrogen Bromide: A Deep Dive into Its Lewis Structure
Unlocking the Chemistry Behind Hydrogen Bromide: A Deep Dive into Its Lewis Structure
Hydrogen bromide (HBr) stands as a quintessential compound in inorganic chemistry, renowned for its strong acidity, gaseous volatility, and pivotal role in human-made compounds like hydrogen bromide liquid (Br₂H) and organic synthesis. Understanding its molecular architecture through Lewis structure provides the foundational insight into its bonds, polarity, and reactivity—elements critical to both academic study and industrial application. The simple diatomic hydrogen bromide molecule, H–Br, belies a nuanced electronic arrangement that defines its chemical behavior, reactivity patterns, and utility in labs and industry.
The Lewis Structure of Hydrogen Bromide: A Clear Electron Map
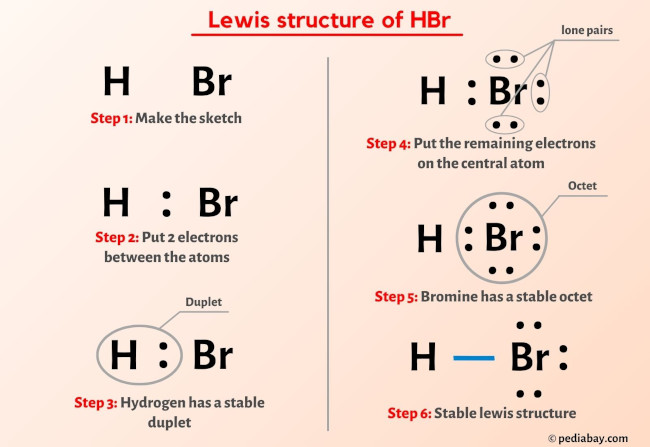
The Lewis structure of hydrogen bromide, though often represented in its most elemental form, reflects the quantum mechanical reality of electron sharing and distribution. At its core, the molecule consists of one hydrogen atom covalently bonded to one bromine atom via a single sigma (σ) bond formed by the overlap of hydrogen’s 1s orbital and bromine’s 4p orbital. This single covalent bond fulfills both atoms’ valence requirements: hydrogen achieves two electrons in its outer shell, fulfilling the duet rule, while bromine completes its seven valence electrons, reaching a stable octet.Unlike ions or more complex molecules, hydrogen bromide exists as a neutral covalent species with minimal formal charges. There is no formal charge on either atom—the formal charge calculation confirms H at 0 and Br at 0, with each atom achieving electronic stability consistent with its group on the periodic table. This stability underpins HBr’s uncommon gaseous state at room temperature; hydrogen bromide is a colorless liquid that evaporates readily, with weak intermolecular forces dominated by London dispersion and dipole-dipole interactions, despite the significant electronegativity difference between H and Br.
>The key feature: a single, polar covalent bond with a dipole moment directed from hydrogen to bromine. > This polarity arises naturally from bromine’s stronger electron-attracting power, creating a partial negative charge (δ⁻) on bromine and a partial positive charge (δ⁺) on hydrogen—a foundational factor in its solubility in water, reactivity in acid-base chemistry, and ability to form hydronium ions when dissolved.
Beyond basic bonding, the Lewis structure illustrates how hydrogen bromide’s simplicity belies functional complexity.
Although the molecule is linear and symmetric, the electronegativity disparity between H (2.20) and Br (2.96) enables a pronounced dipole, influencing its role in proton donation, nucleophilic attack, and industrial synthesis. This electronic polarity makes HBr not just a compound, but a reactive actor in catalytic processes, flame retardant production, and organic halogenation.\n\nHydrogen bromide’s linear geometry, consistent with sp hybridization (though formally unhybridized in simple models), supports efficient orbital overlap and bond formation. It bridges main-group simplicity and application-driven utility, making its structure a cornerstone of intermolecular and reaction dynamics in chemical education and industrial chemistry alike.
Why the Lewis Structure Matters: > For chemists and engineers, the Lewis structure of HBr serves as a predictive tool.
It reveals the molecule’s reactivity tendencies—such as its ability to act as a proton donor in Brønsted acid systems—and informs safer handling protocols due to its fuming nature and corrosive properties. In industrial settings, this structural insight guides its use in synthesis, where controlled proton transfer drives reactions from alcohols to brominated hydrocarbons. Even in atmospheric chemistry, HBr’s dipole and volatility affect its behavior in gas-phase reactions, contributing to stratospheric processes and chlorine cycling.\n\nHydrogen bromide’s molecular simplicity—one hydrogen, one bromine—belies its profound impact across scientific disciplines.
Its Lewis structure, far from static, dynamically informs bond strength, electron mobility, and chemical intent, anchoring HBr’s enduring role in modern chemistry. From lab bench to manufacturing plant, the clarity offered by this structure shapes how scientists harness this versatile compound with precision, safety, and innovation.



Related Post

Unveiling BMW Customer Care: Your Ultimate Guide to Seamless Service

From Mischief to Magic: The Revolutionary Impact of the Cat in the Hat’s Characters

Is Dr. Alan Mandell Married? Untangling The Public Figure’s Personal And Professional World

Derrick Henry’s Family Ties: Unveiling the Roots Behind the Pro Bourgeois Legacy Through His Brothers and Sisters

