Unlocking Energy Freedom: How Exergonic Processes Power Life and Industry
Unlocking Energy Freedom: How Exergonic Processes Power Life and Industry
Picture a world where energy flows effortlessly, not drained from systems but generated through natural, irreversible reactions that fuel both life and technology—this is the essence of exergonic processes, where chemical and physical transformations release usable energy. Defined by exergonic definition as spontaneous reactions that yield net energy release, these processes underpin biology, industrial chemistry, and renewable technologies. From cellular respiration powering our muscles to high-temperature reactions driving nuclear reactors, exergonic phenomena are foundational to efficient energy conversion.
Understanding them reveals not only how energy becomes usable but also how humanity harnesses it in sustainable and scalable ways. <
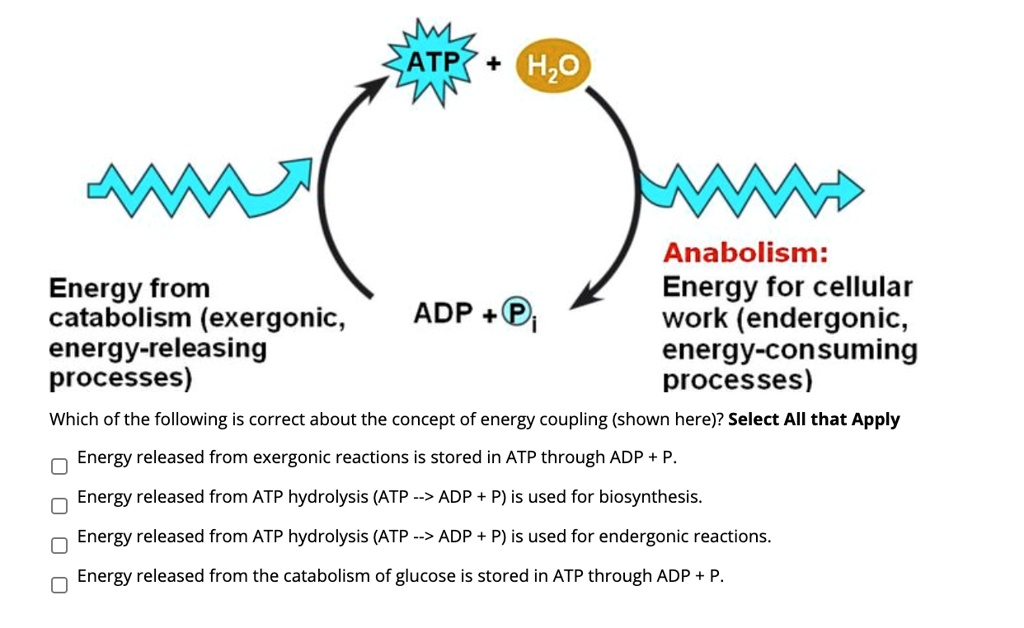
The term exergonic—derived from the Greek *ex* (out) and *ergon* (work)—describes processes where the total free energy of the products is lower than that of the reactants. This energy differential, quantified as a negative change in Gibbs free energy (ΔG < 0), enables spontaneous energy transfer without external input. In biochemical systems, exergonic reactions power cellular energy production: “The breakdown of glucose through glycolysis is a classic exergonic process, releasing roughly 30-32 ATP molecules per molecule under aerobic conditions,” explains Dr.
Elena Marquez, a biochemist at MIT. “Cells exploit the energetic potential of stored chemical bonds to fuel essential functions, from muscle contraction to nerve impulses.” These reactions convert stored molecular energy—such as the phosphate bonds in ATP—into usable forms that drive life’s fundamental activities. Exergonic reactions differ sharply from endergonic processes, which require continuous energy input to proceed.
Biological systems rely almost exclusively on exergonic pathways to maintain efficiency, using redox reactions, hydrolysis, and proton gradients to capture and channel energy. “This selectivity across chemistry and biology is critical,” notes Dr. Raj Patel, energy systems researcher at Stanford.
“Exergonic reactions enable organisms and machines to operate with minimal waste, a principle engineers now emulate in clean energy systems.” <
Marquez. “They deliver efficiencies up to 60%, far exceeding traditional combustion engines, which waste up to 70% of energy as heat.” Industrial catalytic processes also depend on exergonic shifts. In ammonia synthesis—the backbone of fertilizer production—nitrogen and hydrogen molecules react under high pressure and temperature in a highly exergonic reaction that powers global agriculture.
Though requiring intense energy input, the reaction’s overarching thermodynamics reflect exergonic logic: free energy release sustains the process, minimizing net input. Another vital arena is nuclear energy. Fission reactions in reactors involve splitting atomic nuclei—exergonic by nature due to binding energy differences—releasing vast amounts of thermal energy.
“Each fission event converts a fraction of mass into energy via Einstein’s equation E=mc²,” clarifies nuclear physicist Dr. Li Wei. “This exergonic transformation enables gigawatt-scale power generation with minimal fuel.” Renewable technologies increasingly integrate exergonic chemistry too.
Biomass conversion transforms organic matter into biogas or biofuels through controlled, exergonic decomposition. “Microbial digestion of organic waste harnesses natural exergonic pathways to produce methane-rich biogas—a renewable substitute for natural gas,” says Dr. Wei.
“These systems close energy loops sustainably, embodying the exergonic ideal of efficient, low-waste energy extraction.”
Exergonic processes thus emerge as linchpins across biology and technology. They represent nature’s most elegant solution to energy transformation—spontaneous, efficient, and waste-conscious. As global demand for clean, scalable energy grows, harnessing exergonic reactions offers a pathway forward: not through brute force, but through precision, alignment, and deep scientific insight into energy’s hidden potential.
In essence, exergonic processes are the silent architects of modern energy systems.
Their ability to release usable energy spontaneously underpins everything from heartbeat-driven life to grid-scale power plants. Mastery of these reactions bridges fundamental biology and cutting-edge engineering, turning theoretical thermodynamics into tangible, scalable progress. The future of sustainable energy—and efficient industrial design—lies in understanding and applying exergonic principles with clarity and purpose.

Related Post

JenAffleck: Redefining Modern Authorship Through Vulnerability and Craft

A Deep Dive Into Sam Elliott’s Political Soul: From Western Silver to the Pint of Power
What Time Is It in San Diego? Precision, Time Zones, and Real-World Rules

How Old Is The Rizzler? Unraveling the Age and exploded Popularity of a Rising Star in Modern Entertainment

