Tragedy Born from a Single Word: How the Eponym "Hemolysis" Shaped Medical Understanding
Tragedy Born from a Single Word: How the Eponym "Hemolysis" Shaped Medical Understanding
When hospitals treat disease, every term matters—none more precisely than “hemolysis,” a clinical eponym rooted in Greek roots that defines a life-threatening process with profound diagnostic and therapeutic implications. This term, derived from ἡμο- (blood, literally "around the blood") andWestern wooden image of hemolysis in red fluid staining tissue—represents not just a laboratory finding, but a cornerstone in hematology. From early battlefields medicine to modern critical care, the study of hemolysis has shaped how clinicians identify red blood cell destruction, respond to haemolytic crises, and develop life-saving interventions.
Its legacy, embedded in clinical vocabulary, reveals how language refines medical science—and how a single eponym can transform patient outcomes.
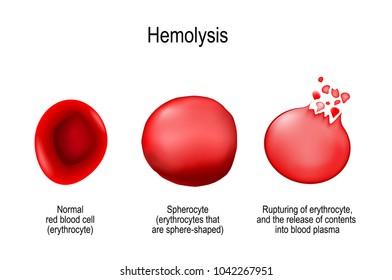
At its core, hemolysis refers to the rupture of red blood cells (RBCs), releasing hemoglobin into the plasma—a process with cascading physiological consequences. While often benign in controlled settings, such as in new-born screening or vaccine monitoring, uncontrolled hemolysis becomes a medical emergency, known as hemolytic disease or hemolytic crisis. The term gained formal medical use in the late 19th century, emerging from German and French clinical studies.
“Hemolysis,” coined to describe the precise cellular rupture, quickly became indispensable in describing conditions like sickle cell disease, transfusion reactions, and autoimmune hemolytic anemia. “Understanding hemolysis is foundational,” notes Dr. Elena Marquez, a senior hematologist at Johns Hopkins Hospital.
“It bridges basic science and clinical action—without it, diagnosing and managing membrane defects would remain largely speculative.”
The Mechanism: Hydrolysis of Red Blood Cell Membranes
To grasp hemolysis, one must understand its cellular mechanics. RBC membranes, stabilized by cytoskeletal proteins like spectrin and ankyrin, maintain shape and flexibility despite constant shear stress in circulation. When these structural supports fail—triggered by oxidative stress, immune attack, or genetic defects—the cell membrane destabilizes.
This rupture releases hemoglobin, free to bind nitric oxide and cause vasoconstriction, and induces jaundice via unconjugated bilirubin. The process unfolds in stages: initial membrane blebbing, followed by echinocyte (b verre-like) formation, and eventual schistocyte fragmentation in severe trauma or microangiopathy. “Each stage marks a shift in clinical severity,” explains Dr.
Rajiv Patel, a transfusion medicine specialist. “Microscopic assessment of RBC shape under high magnification remains a rapid diagnostic shortcut—even before blood smears are analyzed.”
Three primary eponym-dogeneous mechanisms drive hemolysis: 1. **Immune-mediated hemolysis**, as in autoimmune hemolytic anemia where autoantibodies trigger complement activation; 2.
**Non-immune destructive forces**, including mechanical trauma from prosthetic valves or severe sickle cell crises; 3. **Oxidative injury to hemoglobin**, seen in G6PD deficiency when red cells succumb to reactive oxygen species. Each invokes distinct diagnostic criteria, treatment protocols, and prognostic expectations—all anchored in the term hemolysis.
Clinical Impact: From Diagnosis to Critical Care
In modern medicine, hemolysis is more than a pathological curiosity—it’s a clinical alarm.
In the emergency department, rapid detection of hemolytic symptoms guides immediate intervention: saline hydration, corticosteroids for autoimmune causes, or exchange transfusion in acute overload. Blood tests measure lactate dehydrogenase (LDH), indirect bilirubin, and haptoglobin—biomarkers directly tied to hemolysis intensity. “We use hemolysis indices to gauge response to therapy,” states Dr.
Lena Torres, a critical care physician. “A drop in LDH post-treatment confirms efficacy; persistent elevation signals ongoing damage.”
Highlighting key cases, trisomy 21 patients remain at risk for hemolytic disease due to IgG antibody carrying, a complication precisely defined—and managed—through hemolysis monitoring. Similarly, neonates with Rh or ABO incompatibility face acute hemolytic transfusion disease, where clinical hyperbilirubinemia is both symptom and hazard.
“Timely diagnosis of hemolysis in newborns can prevent kernicterus and long-term neurodevelopmental injury,” underscores Dr. Torres. “The term isn’t just a label—it’s a call to action.”
Prognosis and Innovation: Evolving with the Eponym
The term hemolysis has not only clarified clinical understanding but also spurred innovation.
Blood banks now screen donated RBCs rigorously for hemoglobin stability, leveraging hemolysis models to predict shelf-life and safety. New therapies, including monoclonal antibodies targeting complement proteins in immune hemolysis, stem directly from decades of research framed by this eponym. “Eponyms like hemolysis act as cognitive anchors—they unify research, teaching, and practice,” notes Professor Amina Diallo, a medical historian specializing in clinical terminology.
“They give names to invisible processes, turning biological chaos into actionable knowledge.”
Beyond individual cases, “hemolysis” embodies a paradigm: how precise language transforms observation into intervention. In hematology and critical care, this single term—rooted in ancient Greek and refined through modern science—remains central. Every time a patient’s blood smear shows echinocytes or a lab report flags elevated LDH, hemolysis is not just mentioned; it becomes the roadmap guiding diagnosis, treatment, and hope.
In the field where seconds save lives, the eponym “hemolysis” endures not as a relic, but as a living standard—proof that medical precision saves lives.



Related Post
What Are Prime Numbers? The Building Blocks of Mathematics—And Why They Matter

The 24 Game Cards Online PDF: Unlocking Strategic Depth in Digital Card Gaming

Jeffrey Sebelia: Mastering Fire and Ice in Extreme Circus Arts

StudentsForADemocraticSociety: Forging Youth Voice in the Fight for Democratic Renewal

