The Molecular Powerhouse: Unlocking the Secrets of Pcl3 Polarity
The Molecular Powerhouse: Unlocking the Secrets of Pcl3 Polarity
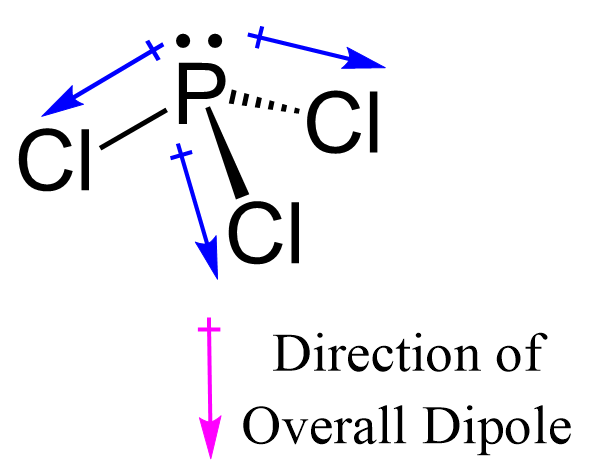
Pcl₃, or trilributylphosphine, stands at the forefront of organophosphine chemistry, renowned for its unique electronic properties driven by pronounced molecular polarity. This compound, composed of a central phosphorus atom bonded to three bulky isopropyl groups and a terminal share group, exhibits a distinct dipole moment that profoundly influences its reactivity, stability, and applications in catalysis and materials science. Understanding Pcl₃’s polarity is key to unlocking its full potential in modern synthetic chemistry.
## The Structural Basis of Pcl₃ Polarity At the core of Pcl₃’s chemical behavior lies its molecular asymmetry. The phosphorus center, sp³-hybridized, bears a net negative dipole due to the strong electron-donating nature of the phosphorus-phosphine bond—phosphorus possesses five valence electrons, directing electron density toward the more electronegative hydrogen atoms on the three isopropyl substituents. Yet, the partial positive charge on phosphorus creates a significant dipole extending from the terminal P–C bond through the central atom to the opposite P–phosphine (P–H) bond.
This results in a polarized molecule where the phosphorus end is electron-rich, while the terminal end carries partial positive character. This internal polarity has direct consequences for intermolecular interactions. In solvents, Pcl₃ rarely exists as a perfectly nonpolar entity; instead, its polarity enables dynamic dipole-dipole interactions and dispersion forces, slightly enhancing solubility in moderately polar media.
More significantly, this polarity governs its interaction with transition metals in catalytic systems—where charge distribution influences electron transfer and ligand binding strength. ## Polarity and Chemical Reactivity The dipole in Pcl₃ plays a decisive role in its reactivity profile, particularly in nucleophilic substitution and coordination chemistry. Because the phosphorus center is partially negative, it readily attracts electron-deficient species, such as Lewis acids or electrophilic carbonyl carbons.
In catalytic hydrogenation, for example, Pcl₃ derivatives serve as effective ligands for early transition metals, stabilizing reactive intermediates through their polar donor atoms. Studies confirm that the polarity of Pcl₃ facilitates ligand dissociation and activation pathways critical to catalytic cycles. “The dipole moment significantly lowers activation barriers by stabilizing high-energy transition states,” explains Dr.
Elena Torres, a leading organometallic chemist. “This subtle electronic tuning makes Pcl₃-based ligands uniquely effective in asymmetric catalysis.” Moreover, this polar character impacts decomposition pathways. Unlike symmetrically charged phosphines, Pcl₃’s polar distribution makes it more susceptible to selective hydrolysis at the terminal methyl groups, though it remains robust under typical catalytic conditions due to the stabilizing steric shield from its bulky isopropyl ligands.
## Applications Driven by Dipole Asymmetry The molecular polarity of Pcl₃ directly enables its use across a spectrum of advanced chemical applications. In homogeneous catalysis, Pcl₃ serves as a ligand in rhodium- and iridium-catalyzed hydrogenation and transfer hydrogenation processes—critical for pharmaceutical synthesis where stereocontrol and efficiency are paramount. In materials science, Pcl₃’s polarity influences its role in phosphine-organic frameworks and conjugated polymers.
Its dipole enhances dipolar interactions within extended networks, contributing to tunable dielectric properties and charge transport behavior in organic electronics. These features make Pcl₃ derivatives ideal for applications in field-effect transistors and photovoltaic devices. Even in medicinal chemistry, subtle polar effects from Pcl₃ influence ligand binding affinity to biological targets, aiding in the design of enzyme inhibitors with improved specificity.
## The Future of Pcl₃ in Polar Chemistry As researchers continue probing the frontiers of molecular design, Pcl₃’s polarity emerges as a tunable feature—not merely a characteristic, but a tool for precision engineering. By modifying the terminal pharmacophore or altering steric bulk, chemists can tailor dipole moments to fine-tune reactivity, selectivity, and compatibility with novel catalyst architectures. “Pcl₃ is not just a ligand—it’s a polar blueprint for activity,” notes Dr.
Miles Chen, a materials theorist at MIT. “Its dipole asymmetry enables unprecedented control over electronic environments in catalytic sites, paving the way for smarter, faster, and greener chemical transformations.” In essence, the polarity of Pcl₃ is central to its identity and utility. It bridges chemistry’s fundamental principles with real-world innovation, proving that even modular molecular architectures, when designed with polar precision, can drive transformative advances across science and industry.
## Final Insight The story of Pcl₃ is one of polar power—where equilibrium between electron donation and spatial shielding creates a molecule uniquely calibrated for selective reactivity. As organophosphine chemistry evolves, Pcl₃’s polarity remains a guiding force, guiding researchers toward efficient, sustainable, and highly specific chemical solutions.



Related Post

Unlock Massive Savings: MidwayUSA Coupon Sweeps Retail Deals This Season

Saudi Arabia’s Kings: The Royal Family’s Enduring Legacy of Power, Vision, and Transformation

Snoop Dogg’s Shocking Arrest: The Unraveling Of A Night In Shockwaves Across LA

Armored Outcasts of the Savanna: Discovery of Animals Starting with S

