The Limiting Reactant: The Silent Determinant of Chemical Success
The Limiting Reactant: The Silent Determinant of Chemical Success
In the intricate dance of chemical reactions, one molecule quietly dictates outcome—often without a spotlight. This is the limiting reactant, a fundamental concept that determines how much product can be formed in any given reaction. When chemists speak of limiting reactants, they refer to the substance that runs out first, thereby caps the reaction’s capacity and shapes the final yield.
Understanding this principle is not just academic—it’s the backbone of efficient process design in pharmaceuticals, manufacturing, and environmental science. As chemist Dr. Elena Torres notes, “The limiting reactant is the unsung hero of stoichiometry—without knowing it, you’re building a house on shifting sands.”
Defining the limiting reactant requires precision: it is the reactant consumed completely before others, leaving excess materials unutilized.
This contrasts with excess reactants, which remain after the reaction ceases. The moment a limiting reactant is exhausted halts product formation, making it the decisive factor in yield calculations. “In every balanced equation,” explains professor Mark Chen, “it’s the limiting reactant that sets the upper limit on output—no amount of unspent reagent can create more product.” This principle governs every reaction from the synthesis of water (H₂ + O₂ → 2H₂O) to industrial chemical manufacturing where efficiency directly impacts cost and scalability.
The Mechanics: How Limiting Reactants Govern Reaction Yields
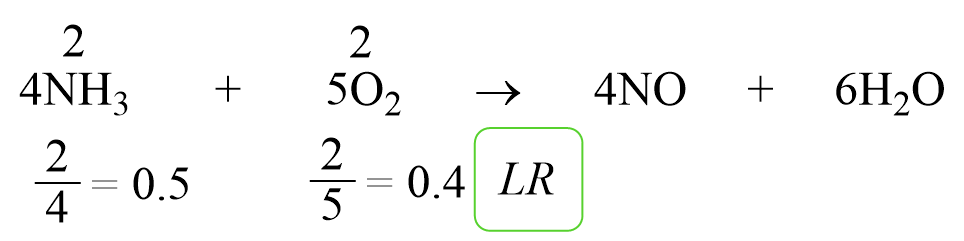
At the core of chemical stoichiometry lies the law of conservation of mass, ensuring atoms are neither created nor destroyed. But practical yields diverge from theoretical maximums due to reactant availability. The limiting reactant controls the reaction’s extent, defined by the mole ratio in a balanced equation.When reactants combine in fixed proportions, one will be used up first, defining the maximum quantity of product formed. To determine the limiting reactant, follow this structured approach: - **Step 1: Identify moles** of each reactant using measured masses and molar masses. - **Step 2: Compare** these values to the balanced equation’s coefficients.
- **Step 3: Calculate** how much product each reactant could theoretically yield. - **Step 4: Select the smallest yield**—this reactant limits the reaction. For example, in the combustion of methane: CH₄ + 2O₂ → CO₂ + 2H₂O.
Suppose 10 grams of methane (CH₄) react with 64 grams of oxygen (O₂). First, convert to moles: - CH₄: 10 g ÷ 16 g/mol = 0.625 mol - O₂: 64 g ÷ 32 g/mol = 2.0 mol Balanced equation requires 2 moles O₂ per 1 mole CH₄. For 0.625 mol CH₄, 1.25 mol O₂ is needed.
Since only 2.0 mol O₂ is available—more than required—the limiting reactant is methane, capping the reaction. Thus, methane dictates how much carbon dioxide and water form.
This dynamic reveals a critical insight: the limiting reactant often exists in the smallest quantity by mass, not necessarily by formula.
Excess reagent may remain unreacted, representing lost raw materials and potential waste. In industrial settings—where margins depend on precision—this knowledge drives cost control and sustainability efforts.
Real-World Applications and Industrial Impact
In pharmaceutical production, where even minor inefficiencies amplify into massive financial loss, identifying the limiting reactant ensures optimal reagent use. A classic case involves the synthesis of aspirin (acetylsalicylic acid) from salicylic acid and acetic anhydride.Miscalculating the limiting component can reduce yield from a target 95% to 60%, scrapring tons of material. Pharmaceutical chemists rigorously screen reactants, using limiting calculations to scale processes safely from lab to factory. Similarly, in environmental engineering, remediation reactions depend on limiting reactants to neutralize pollutants.
For instance, in treating acidic wastewater (H₂SO₄ + 2NaOH → Na₂SO₄ + 2H₂O), if sodium hydroxide runs low, sulfuric acid remains partially unreacted, failing to stabilize the pH. This risk drives process designs that pre-calculate stoichiometric needs, ensuring complete treatment with minimal excess. Chemical engineers apply the limiting reactant principle in reactor design, reaction optimization, and safety planning.
Whether in batch, continuous flow, or catalytic systems, anticipating this constraint prevents runaway reactions and undefined product formation. “Accurate limiting reactant analysis isn’t just about numbers—it’s about risk mitigation, cost control, and environmental stewardship,” asserts Dr. Maria Lopez, senior process chemist.
Failed Reactions and the Cost of Ignoring Limits
When the limiting reactant is mishandled, outcomes range from suboptimal yields to catastrophic failures. In 2010, a major chemical plant experienced an explosion due to an overlooked limiting reactant: excess unreacted hydrogen was mistakenly assumed sufficient, causing an uncontrolled exothermic runaway. Investigations revealed that the original dosing neglected stoichiometric limits, leading to thermal instability.“This incident underscores the existential importance of limiting reactant identification,” cautioned safety expert James Reed. “In high-energy reactions, ignoring this even one reactant can turn chemistry from precise science into catastrophe.” Beyond safety, economic impact is profound. Excess raw material waste increases production costs and environmental burden.
The chemical industry now invests heavily in automated stoichiometric modeling and real-time monitoring systems to prevent such oversights. Software tools simulate reactions dynamically, adjusting inputs based on live reactant availability—turning formulas into living systems of precision.
Understanding the limiting reactant is thus not merely academic—it’s a matter of precision, safety, and sustainability.
From laboratory benchwork to megaton-scale manufacturing, this concept ensures reactions proceed as intended, maximizing yield while minimizing waste. As advanced analytical tools redefine stoichiometric control, the role of the limiting reactant remains central—gu

/simple-experiment-58b5b3325f9b586046bbfa7f.jpg)

Related Post

What Does EOM Mean in Finance? The Critical Timestamp Shaping Markets and Decisions

Why Football’s Young Talent Still Rules the Game: The Full Story on Average Age of Professional Players

The U.S. Constitution: The Living Blueprint That Shaped a Nation

Wetv: Revolutionizing Digital Media with Immersive, Interactive Live Video

