The Chemical Formula for Water: Nature’s Simplest Molecule with Profound Power
The Chemical Formula for Water: Nature’s Simplest Molecule with Profound Power
WhatIsTheChemicalFormulaForWater takes center stage each time we pause to reflect on life’s most essential compound—water. Though its molecular structure may appear deceptively simple, this pairing of hydrogen and oxygen reveals a substance central to biology, climate, and industry. Understanding its chemical formula unlocks deeper insight into water’s unique behavior and universal importance. Water is scientifically defined by its molecular composition, where two hydrogen atoms bond covalently to a single oxygen atom.This simple combination—H₂O—masks a complex array of physical and chemical properties that make water indispensable to Earth’s ecosystems and human survival. The formula H₂O encapsulates more than just atoms; it represents hydrogen and oxygen bound in a precise 2:1 atomic ratio, resulting in a molecule both stable and dynamic.
At the atomic level, the hydrogen-oxygen bond is formed through covalent molecules featuring shared electrons, a strong interaction that gives water remarkable stability and high cohesion.
Each water molecule consists of one oxygen atom with six valence electrons (two bonded to hydrogen, four unpaired) and two hydrogen atoms each contributing one electron to complete the bond. This hybridization supports water’s polarity—a key factor behind its ability to dissolve countless substances and facilitate chemical reactions.
The bonds within water molecules are hydrogen-oxygen covalent bonds, with an unequal electron sharing that creates molecular polarity. Oxygen, being more electronegative, pulls the shared electrons closer, giving the molecule a distinct dipole moment—one end slightly negative and the other slightly positive.
This polar nature enables water to act as a universal solvent, dissolving salts, sugars, gases, and many minerals vital to living systems. The O-H bonds also allow extensive hydrogen bonding, a special intermolecular force responsible for water’s high boiling point, surface tension, and thermal stability.
Beyond its molecular form, water’s chemical identity underpins its role as life’s medium. It drives metabolic processes, transports nutrients, regulates temperature through evaporation and condensation, and shapes geological features through erosion and deposition.
Industries rely on water’s solvent properties, capillary action, and reactive hydrogen bonds in chemical synthesis, waste treatment, and manufacturing. Even climate systems depend on water’s high heat capacity, moderating Earth’s temperatures.
H₂O — the chemical formula—serves not only as a scientific label but as a gateway to understanding why this compound is foundational across disciplines. From hydrology to biochemistry, from environmental science to cosmology, water’s molecular identity defines its behavior and significance.This formula, though brief, encapsulates centuries of scientific discovery: from early elemental theories to modern quantum mechanical models that explain electron distribution and molecular geometry.
While the formula H₂O is a single line of chemical notation, its implications span the microscopic dance of particles to the global cycles shaping our planet. The molecule’s simplicity belies its complexity: hydrogen and oxygen, two of Earth’s most abundant elements, merge to form a substance that sustains ecosystems, fuels biological evolution, and animates human civilization. Each water molecule is a testament to nature’s elegance—few bonds enabling life-sustaining phenomena on a planetary scale.
As scientists continue probing water’s behavior at nanoscale and quantum levels, H₂O remains a benchmark for molecular stability, polarity, and intermolecular interaction.
Its formula, so brief and universal, continues to anchor education, research, and innovation worldwide. In every drop, the chemistry of life unfolds—anchored in the precise ratio of two hydrogen atoms to one oxygen atom, forever echoing nature’s most enduring chemical signature.
This formula is more than notation: it is a key to understanding one of Earth’s most precious resources—water in its purest, most transformative form. Recognizing H₂O’s chemical truth deepens not only scientific literacy but respect for water’s irreplaceable role across nature and society.

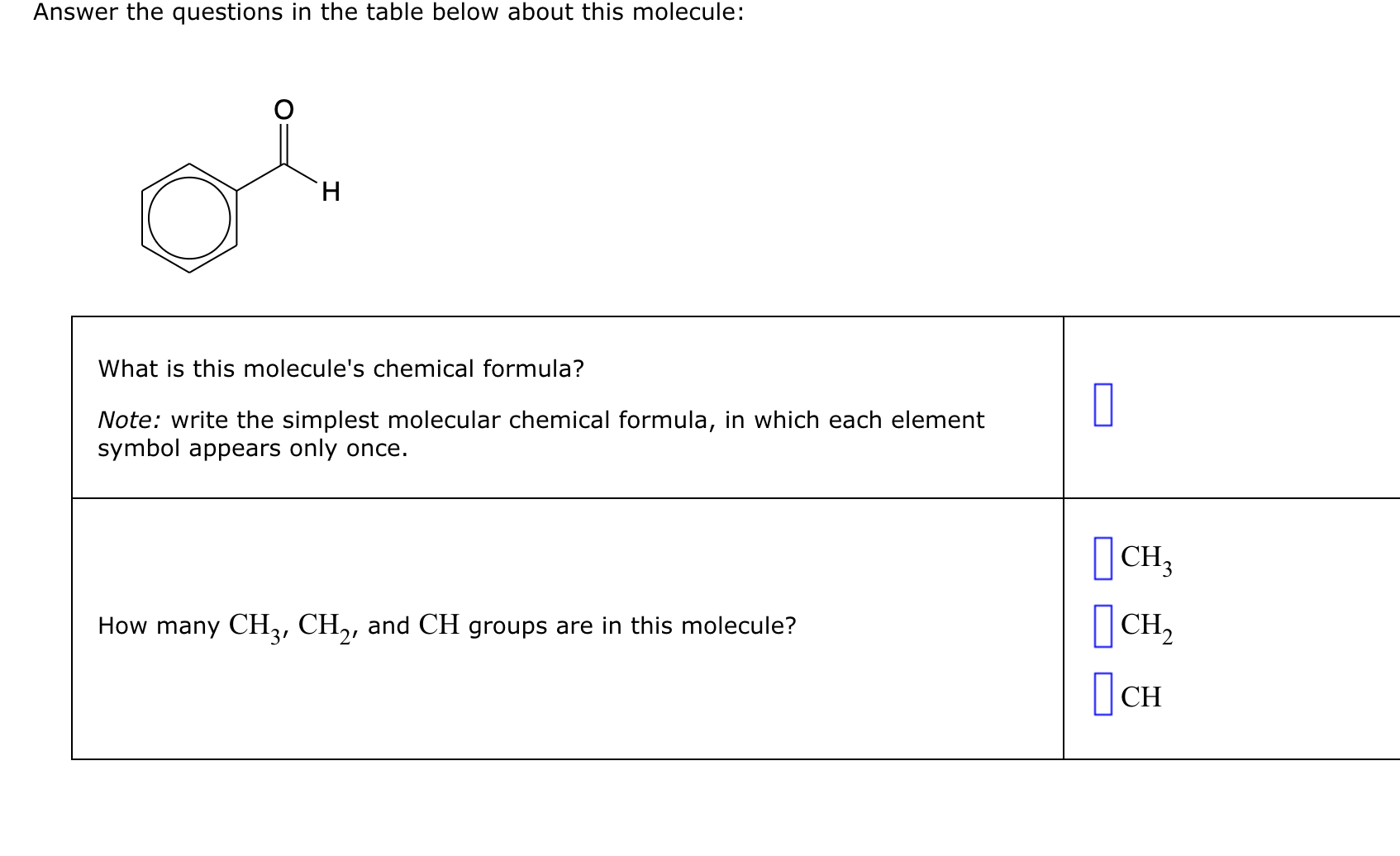
Related Post

Unlocking the Mystery of Human Behavior: Inside Hahn TV Series
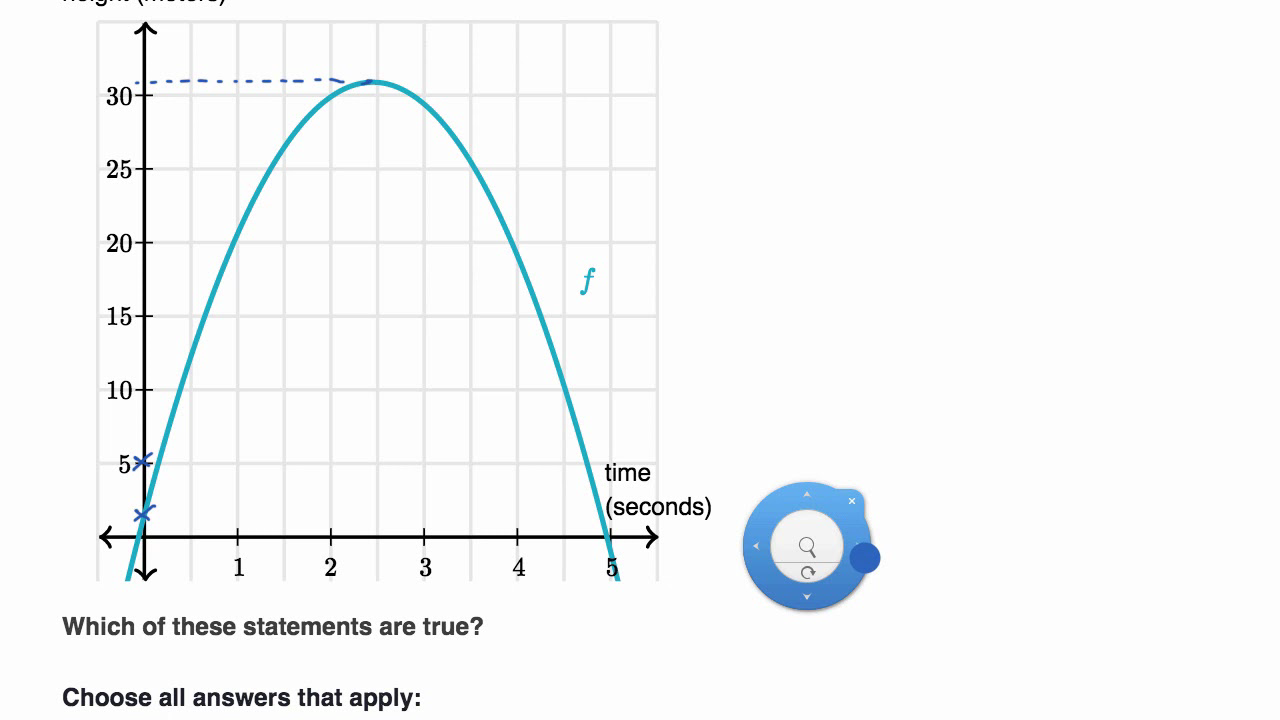
Decode Quadratic Graphs Like a Pro – Key Insights from Khan Academy’s Quadratic Answers

Exit 8: What Happens After The Credits Roll? The Silent Aftermath of Cinema’s Grand Finale

Is the UAE in the Middle East? Unveiling a Region’s Geopolitical Identity

