The Breath of Chemistry: Decoding Boyle’s Law Through Graphical Insights from BoylesLawGraph
The Breath of Chemistry: Decoding Boyle’s Law Through Graphical Insights from BoylesLawGraph
A foundational principle in gas physics, Boyle’s Law reveals a profound relationship between pressure and volume—one that remains as vital today as it was centuries ago. Using real-time visualizations from BoylesLawGraph, the law illuminates how pressure increases inversely with volume when temperature remains constant, forming a cornerstone of thermodynamics and engineering applications. From laboratory experiments to industrial process design, this relationship guides everything from scuba diving safety to HVAC optimization.
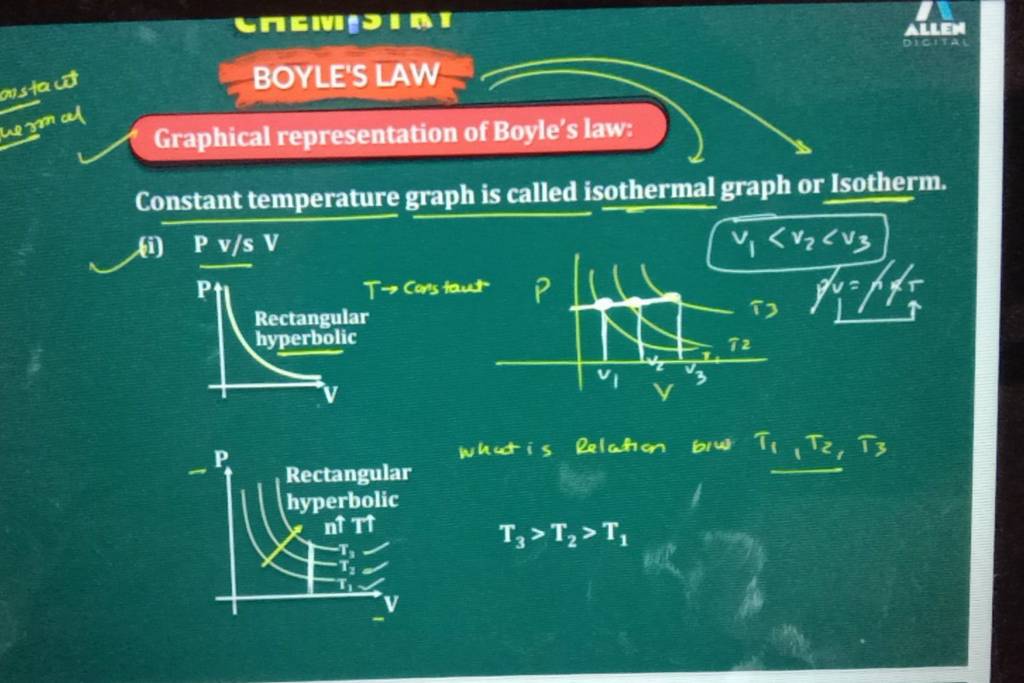
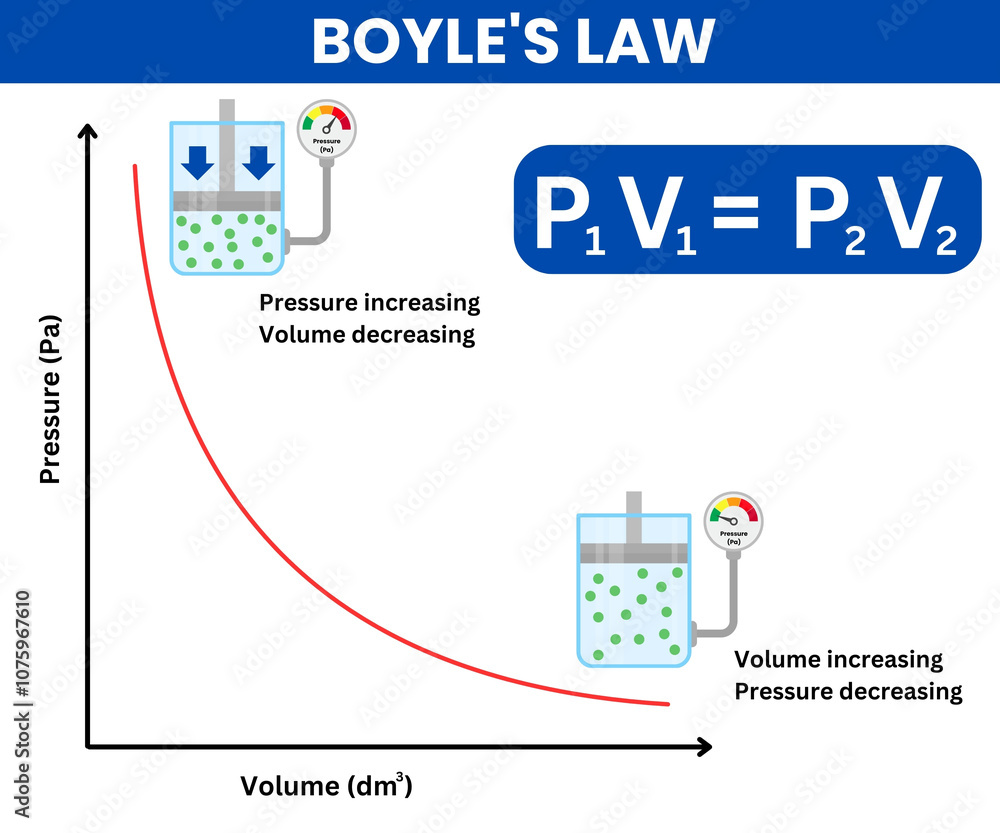
The BoylesLawGraph platform transforms abstract equations into dynamic, intuitive visuals, enabling scientists, engineers, and students to explore the law’s mechanics with unprecedented clarity. Boyle’s Law, named after Robert Boyle, a 17th-century Irish natural philosopher, states: at constant temperature, the pressure of a fixed amount of gas is inversely proportional to its volume. Mathematically expressed as P ∝ 1/V, or more precisely P₁V₁ = P₂V₂, the law emerges from the behavior of gas molecules colliding with container walls.
As volume decreases, molecule collisions intensify, raising pressure; conversely, expanding volume reduces collisions and pressure. This inverse relationship isn’t just a theoretical curiosity—it underpins critical processes in physics and engineering, from compressing air in pneumatic systems to regulating breathing in living organisms.
Visual Mastery: How BoylesLawGraph Brings the Law to Life
Harnessing digital innovation, BoylesLawGraph converts the ideal gas equation into dynamic, interactive graphs that reveal the pressure-volume interplay in real time.Unlike static diagrams, the platform animates how a gas responds as volume shifts under isothermal conditions. Users observe pressure rising sharply when volume shrinks—such as when pushing air into a bicycle pump—and falling steadily as the container expands, like a balloon deflating. These visual revelations demystify the abstract, turning equations into observable trends.
The graph clearly identifies the hyperbola trademark of Boyle’s Law, where each quarter-circle segment represents a proportional inverse relationship. This shape is not merely a pattern—it’s physical evidence that pressure and volume adjust predictably, supporting the law’s validity across samples of ideal gases. Observers note, “Seeing pressure spike tenfold when volume halves—this isn’t just math, it’s nature in motion,” a sentiment echoed across educational forums.
Interactive sliders allow users to manipulate initial volume or pressure, instantly observing how the gas curves adjust while maintaining their product constant. Sudden shifts in slope highlight critical thresholds, reinforcing how sensitive gas behavior is to even small volume changes. Each animation underscores a core principle: the system traces a smooth, predictable curve governed by thermodynamic consistency.
The absence of temperature variation ensures heat transfer effects are isolated, focusing views strictly on pressure-volume dynamics. This rigorous control enhances理解 for both learners and professionals, enabling precise analysis of behavior before expanding into complex real-world condition layers.
Beyond classroom demonstrations, BoylesLawGraph proves indispensable in practical applications.
Consider scuba diving: as divers descend, water pressure increases, compressing the air in tanks and lung cavities. Advanced dive computers use Boyle’s Law to model air volume reduction, warning divers of breathing risks at depth. “Boyle’s Law on a graph is like a diver’s silent navigator,” explains a certified diving instructor, “showing how pressure changes shape every breath under water.”
In HVAC design, engineers rely on Boyle’s Law to predict airflow through compressible duct systems.
Heating and cooling cycles alter volume and pressure; accurate gas behavior modeling prevents inefficiencies and ensures comfort. The graph’s interactive design allows testing multiple scenarios—from seasonal temperature swings to emergency pressure spikes—without costly physical trials. “With BoylesLawGraph, we simulate every possible variation,” says mechanical systems specialist Dr.
Elena Torres, “then fine-tune controls for peak performance.”
The platform also illuminates biological processes. In physics of respiration, Boyle’s Law explains inhalation and exhalation: the diaphragm’s movement expands the thoracic cavity, halving lung volume and doubling internal pressure, drawing air in. Exhalation reverses the cycle—volume rises, pressure drops, and air flows out.
These biological “breathworks” unfold visibly on the graph, transforming physiology into a story of pressure and volume in motion. “Watching the law during breathing is awe-inspiring,” notes a biomedical researcher, “this graph makes the invisible visible, grounding medical understanding in observable physics.”
Interactivity remains a defining strength. Users can overlay data points, annotate volume-pressure transitions, and compare dry air to real gases like oxygen or CO₂, revealing subtle deviations from ideal behavior at high pressures or low temperatures—an essential for industrial gas applications.
Real-time recalibration shows how volume changes ripple through pressure curves, reinforcing causal links. This dynamic engagement fosters deeper comprehension, moving beyond memorization to intuitive grasp. Highlighting common misconceptions, the platform clarifies limitations—Boyle’s Law assumes ideal gases and isothermal conditions, excluding real-world complexities like gas intermolecular forces or temperature shifts.
Yet within its scope, the graph delivers unmatched clarity, acting as both a teaching tool and analytical resource. Educators emphasize, “Visualizing how pressure explodes from half a volume and double pressure makes Boyle’s Law unforgettable.”
In an era of digital science communication, BoylesLawGraph stands out as a bridge between theoretical physics and experiential learning. Its ability to render abstract gas laws into vivid, manipulable visuals transforms how audiences connect with fundamental science.
From high school classrooms to advanced engineering design studios, the platform proves that physics need not be opaque—when presented dynamically, the language of pressure and volume becomes accessible, compelling, and deeply relevant to real-world problems.
The marriage of Boyle’s Law and graphical interactivity, as showcased by BoylesLawGraph, transforms education and innovation alike. By converting equations into observable phenomena, it reveals not just how gases behave, but why—making one of chemistry’s oldest truths the clearest and most impactful science of our time.

Related Post

Top 10 Mobile Esports Titles Dominating iOS in 2024

Top Interior Design Schools in Norway: Where Scandinavian Elegance Meets Creative Innovation

The Legacy Of Marlon Brando And Timothy Gahan Brando: A Family Tapestry Woven Through Commitment, Charisma, and Contradiction

All About Drew Carey: The Comedy Genius Behind The Show and His Enduring Legacy

