N2 Molar Weight: The Foundational Benchmark in Modern Science and Industry
N2 Molar Weight: The Foundational Benchmark in Modern Science and Industry
At just 28.0134 grams per mole, N2 stands as a cornerstone in chemistry, serving as both a fundamental reference and a critical indicator in scientific and industrial applications. Defined by its precise molar mass, nitrogen’s uniformity underpins countless chemical processes, analytical standards, and quality controls—making it indispensable in research, manufacturing, and environmental monitoring. Understanding its molar weight is not merely an academic exercise; it is a gateway to interpreting reaction stoichiometry, ensuring process accuracy, and advancing innovation across fields.
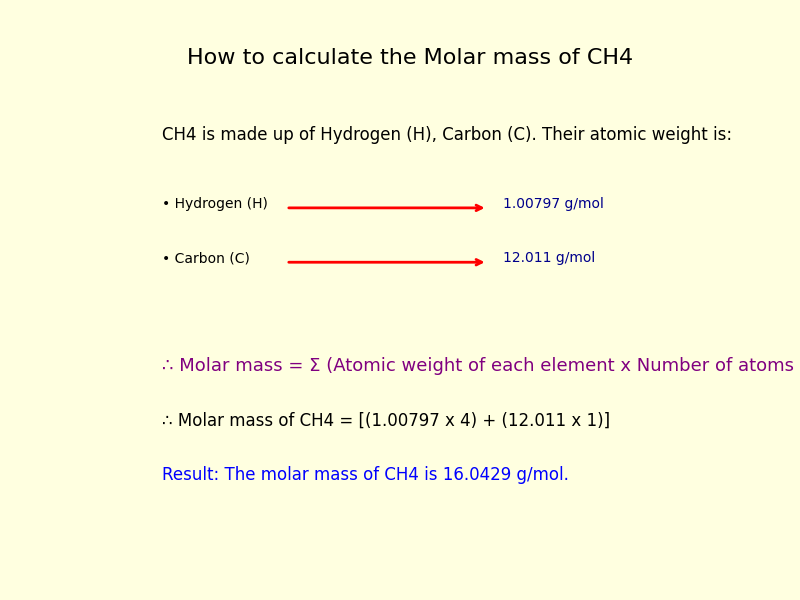
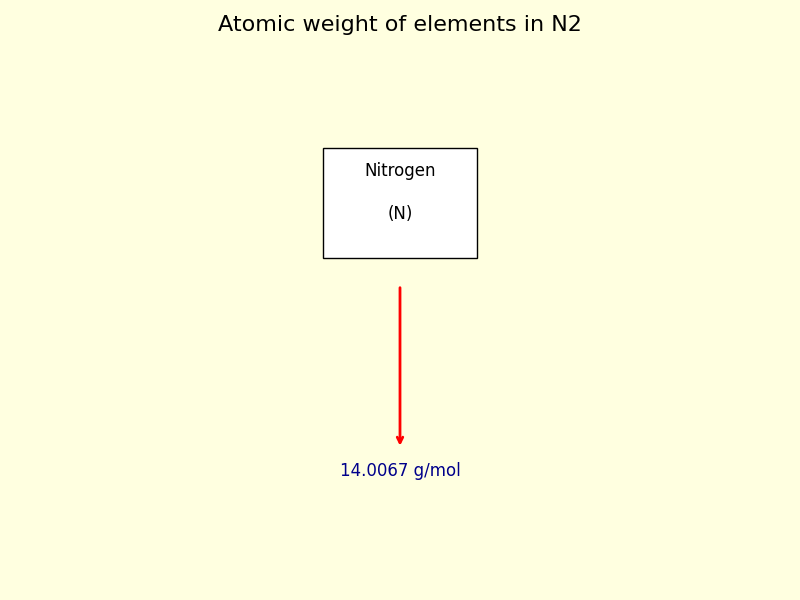
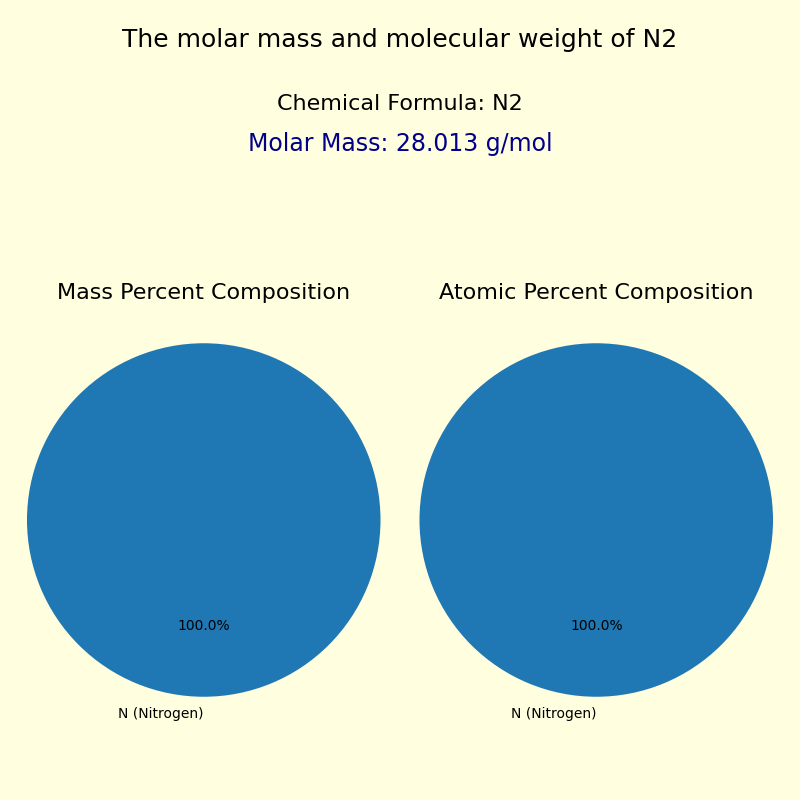
The molar mass of N2, calculated from its atomic weight (14.0067 g/mol for nitrogen, doubling it due to diatomic structure), is universally accepted as 28.0134 g/mol.
This value, derived from Avogadro’s number and precise atomic data, is rigidly standardized across scientific disciplines. "N2’s molar weight is not just a number—it’s the backbone of molecular computations," notes Dr. Elena Rostova, a physical chemist at the Institute of Analytical Sciences.
"From lab-scale experiments to large-scale industrial reactors, every calculation hinges on its exactness."
Why Molar Mass Matters: The Backbone of Chemical Precision
In chemistry, molar mass serves as the bridge between mass and molecular scale. For nitrogen gas, its molar weight enables scientists to translate measurable quantities—grams or kilograms—into the number of molecules, ensuring reproducibility and reliability. This precision underpins: • Stoichiometric Calculations: Whether synthesizing ammonia via the Haber process or determining fertilizer ratios, N2’s known molar mass allows exact molar ratios between reactants and products.
• Gas Law Applications: Ideal gas equations (PV = nRT) depend on molar volume; knowing N2’s molar mass ensures accurate conversions between volume, pressure, and temperature in both lab and engineering contexts. • Analytical Integrity: In spectroscopy and chromatography, reference compounds must have certified molar values to validate instrument performance and data accuracy. N2, often used as a standard gas mixture, ensures traceability across labs worldwide.
The Industrial and Environmental Relevance of N2 Molar Weight
Beyond the laboratory, N2’s molar weight governs critical industrial and environmental systems.
Selecting appropriate equipment for gas handling, storage, and transport relies on density calculations rooted in molar mass. For example, the density of nitrogen at standard conditions—approximately 1.149 g/L—is derived directly from its molar mass, guiding tank sizing and pipeline design. In environmental science, nitrogen builds the foundation of biogeochemical cycles.
Accurately modeling nitrogen fluxes in soil, water, and atmosphere demands precise knowledge of N2’s molar weight to interpret isotope ratios, trace pollutant dispersion, and evaluate ecosystem health. "Without a consistent N2 reference, climate models would lack the accuracy needed to predict nitrogen-related phenomena," explains Dr. Marcus Telford, a geochemist at the Global Environmental Monitoring Institute.
"Its molar mass is non-negotiable for data integrity."
Applications Across Science and Technology
N2’s molar weight finds utility in diverse technical domains.
- Rocket Propulsion: Nitrogen is increasingly used in cryogenic oxidizer mixtures for advanced propulsion systems. Its molar mass influences thrust calculations and combustion efficiency, enabling precise fuel-oxidizer ratios critical for aerospace performance.
- Semiconductor Manufacturing: High-purity nitrogen purges prevent oxidation in sensitive fabrication environments. Molar mass ensures accurate gas flow control, differentiating trace nitrogen from contaminants that could compromise chip yield.
- Medical Gas Systems: Though less common than oxygen, dry nitrogen is vital in respiratory therapy and medical gas mixtures.
Certified molar values guarantee that delivery systems maintain precise concentrations essential for patient safety.
Each application hinges on a single, unchanging value: 28.0134 g/mol. This consistency allows global standardization—from university labs in Tokyo to production lines in Detroit—ensuring data interoperability and process reliability.
Ensuring Accuracy: Traceability and Certification in N2 Standards
Scientific and industrial accuracy demands more than a defined molar mass—it requires traceability. Certified reference materials (CRMs), such as NIST-traceable nitrogen gas, validate N2’s molar weight against international standards.
Laboratories storing reference gases undergo rigorous calibration, using gravimetric and volumetric methods to confirm concentration and purity. "Traceability is the silent pillar of confidence," states Dr. Rostova.
"When an engineer in Shanghai verifies a reactor’s nitrogen feed using a certified CRM, they trust the value is rooted in a globally recognized molar baseline."
Moreover, evolving metrology practices continue to refine how N2’s molar weight is distributed and validated. Modern laser absorption spectroscopy and quantum-based measurements now support ultra-precise determinations, reinforcing its role as a metrological anchor. These advances ensure that while technology evolves, the foundation remains rock-solid.
The Future of Nitrogen in Science and Industry
As industries push toward sustainability and precision, N2’s role is expanding.
Carbon capture technologies increasingly rely on nitrogen-purified streams to separate CO₂ efficiently. Green hydrogen production uses inert nitrogen atmospheres to stabilize electrolysis processes. Meanwhile, quantum computing and advanced materials science depend on ultra-pure nitrogen environments where even minor deviations could alter outcomes.
>"The molar weight of N2 is more than a static number—it’s a living standard shaping tomorrow’s breakthroughs," asserts Dr. Telford. "As our tools grow more sophisticated, this value remains our constant anchor." In research, industry, and environmental stewardship alike, N2’s molar weight endures as a silent but vital force—everywhere precision matters.
Related Post

From Comedy Stups to Hollywood Stardom: The Surprising Talent Behind the Hangover Cast

How McDo’s Breakfast Meal Time Redefines Morning Convenience for Busy Lifestyles

Christian Science Society of Dixon: Healing with Faith, Community, and Spiritual Clarity

How Voipoffice Redefines Communication: Fusion of Innovation, Stories, and Cutting-Edge Tech

