Mastering Molecular Shapes: How Bond Angles Dictate the Geometry of Life at the Atomic Scale
Mastering Molecular Shapes: How Bond Angles Dictate the Geometry of Life at the Atomic Scale
In the invisible world of atoms, geometry governs function. From the simplest water molecule to complex protein structures, the precise angles between chemical bonds determine reactivity, stability, and biological activity. A single touch — a lone pair of electrons or a change in hybridization — can shift bond angles, altering molecular behavior in profound ways.
At the center of this invisible design is the Bond Angle Chart — a powerful tool that reveals the three-dimensional architecture of molecules, enabling scientists to predict properties, design drugs, and engineer new materials. By decoding these angular relationships, researchers unlock secrets behind molecular behavior across chemistry, pharmacology, and materials science. The bond angle, defined as the angle between two covalent bonds meeting at a central atom, is far more than a geometric curiosity — it is a fingerprint of molecular identity.
Major publications have highlighted the bond angle chart’s role as a foundational guide in structural chemistry, illustrating how deviations from ideal angles signal electronic stress and reactivity shifts. For instance, while hydrogen-oxygen-carbon in water naturally adopts a bent structure with a bond angle near 104.5°, deviations due to lone pairs distort symmetry and influence hydrogen bonding strength.
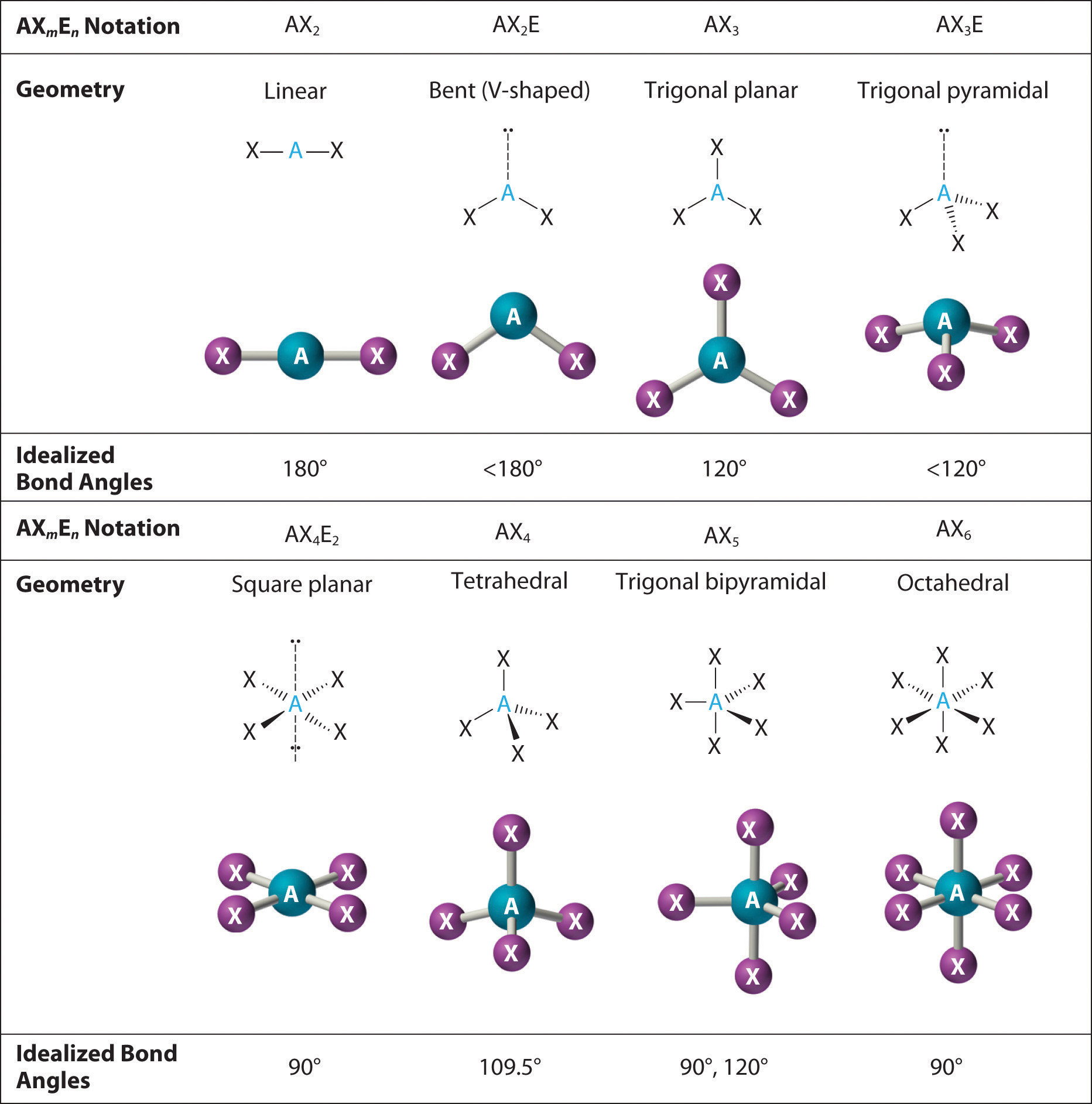
Central to understanding molecular geometry is VSEPR theory — Valence Shell Electron Pair Repulsion — which predicts molecular shapes by minimizing electron pair repulsion around a central atom.
This model explains why methane (hydrocarbon) assumes a perfect tetrahedral angle of 109.5°, while ammonia gains a trigonal pyramidal shape with a bond angle slightly less than 107° due to lone pair repulsion. Beyond simple molecules, Bond Angle Charts map the nuanced distortions seen in more complex systems, including bent, trigonal planar, and seesaw geometries, each telling a unique story of electron distribution and spatial constraints. Quoting chemist Dr.
Elena Moreau of ETH Zurich, “Bond angle measurements are the quantitative voice of molecular architecture. They bridge theory and observation, allowing us to translate electron pairs into real-world shapes — a clarity critical for drug design and material innovation.” Her insight underscores the chart’s value: not just as a diagram, but as a diagnostic tool revealing how geometry shapes chemistry.
The standard bond angle references on any Bond Angle Chart typically reflect key molecular geometries derived from idealized electron domain arrangements.
For example: - Linear molecules (e.g., BE, CO₂) exhibit bond angles of 180°. - Triangular planar (e.g., BF₃) settle near 120°. - Trigonal pyramidal structures (e.g., NH₃) average around 107°.
- Bent (or angular) geometries, like water (104.5°) or sulfur tetrafluoride (104.5°), fall slightly lower due to lone pair repulsion. These values are not arbitrary — they stem from computational models and spectroscopic validation, ensuring consistency across applications. As molecular structures grow more complex, subtle angular shifts become critical indicators of conformational changes, catalytic activity, or binding affinity.
Modern Bond Angle Charts integrate advanced data from X-ray crystallography, neutron diffraction, and quantum mechanical calculations, enabling precise angular determination even in challenging environments. For example, in enzyme active sites, precise bond angles dictate substrate orientation and transition state stabilization. Similarly, in semiconductor nanomaterials, bond angle distortions affect electronic band gaps and charge transport properties.
ęThe bond angle chart thus serves a dual role: as an educational tool for learners grasping molecular geometry, and as a professional reference for researchers navigating complex structural problems. It enables chemists to visualize how substituent effects, hybridization, and external stimuli — such as solvent polarity or temperature — perturb ideal angles, offering predictive insight into molecular behavior.
Real-world applications testify to the chart’s indispensability.
In pharmaceutical development, understanding bond angles around functional groups helps design molecules with optimal receptor binding. For instance, slight angular adjustments in antiviral agents can dramatically enhance efficacy by improving fit within viral enzyme pockets. In materials science, designers manipulate bond angles in polymers and metal-organic frameworks (MOFs) to tune porosity, stability, and catalytic performance — all traceable to precise angular parameters.
Groups working on green chemistry embrace the Bond Angle Chart to minimize trial-and-error by predicting ideal geometries during synthesis, accelerating discovery cycles and reducing environmental impact. Its utility extends to fields as diverse as atmospheric chemistry, where bond angle distortions in trace radicals influence ozone depletion, and synthetic organic chemistry, where conformational control is key to stereoselective reactions.
The bond angle chart is more than a static illustration — it is a dynamic lens through which the molecular world is decoded.
It reveals how a mere 1–2 degree shift can transform a molecule’s reactivity, symmetry, and function. From catalysis to drug delivery, mastery of bond angles enables scientists to shape chemistry with unprecedented precision. As experimental and computational methods advance, this tool evolves, ensuring that the geometry of molecules remains at the heart of scientific innovation.
In the intricate dance of atoms, bond angles define the steps. The Bond Angle Chart stands as both map and compass — guiding discovery through the three-dimensional landscape where chemistry truly unfolds.



Related Post

Project Blood Strike: Mastering The 3-Finger HUD Layout for Victory in Real-Time Combat

Rite Aid Ice Cream Alley Closes: Here’s What You Need to Know

World Series Game 6: Can the Aftermath Decide the Champion?

Ikatmon Fruit: Decoding the Scientific Secrets and Cultural Significance of Ikatmon x Sativa

