How Many Valence Electrons Does Sulfur Have? The Key to Its Chemical Behavior
How Many Valence Electrons Does Sulfur Have? The Key to Its Chemical Behavior
Sulfur, a fundamental element in chemistry, holds a unique position in the periodic table due to its distinctive electron configuration—particularly its four valence electrons. Understanding how many valence electrons sulfur possesses is not just a matter of atomic structure; it directly explains the element’s reactivity, bonding preferences, and role in both natural and industrial chemical processes. With six electrons in its outermost shell, sulfur’s electron count governs its ability to form stable compounds, exhibit multiple oxidation states, and participate in a wide range of biochemical and geological transformations.
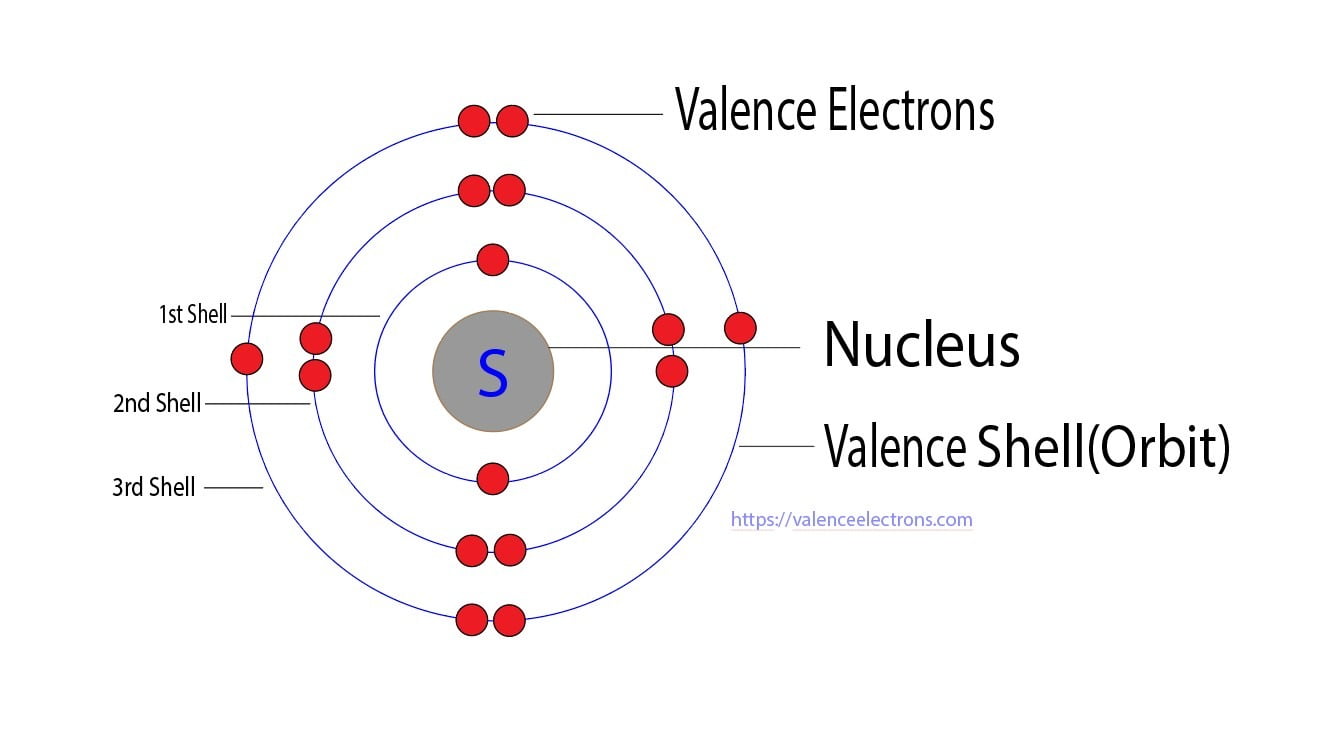
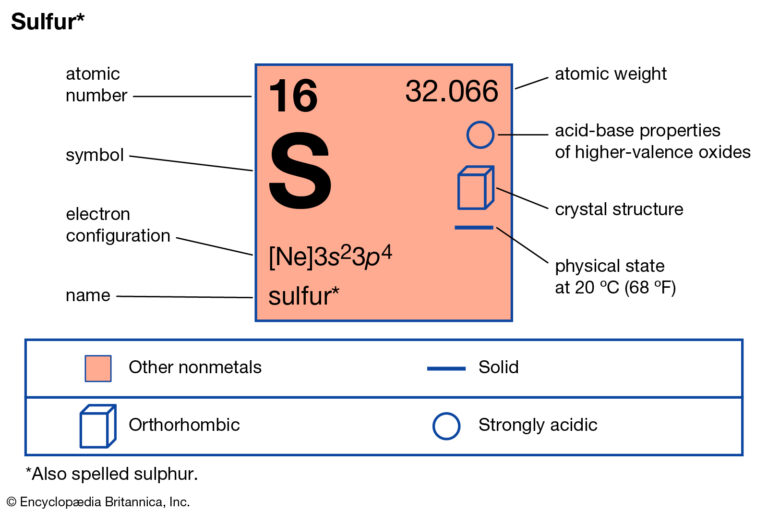
Understanding valence electrons requires a look at sulfur’s position in Group 16 of the periodic table—commonly known as the chalcogens. These elements, including oxygen, selenium, and tellurium, share a consistent pattern of having six valence electrons, which defines their tendency to gain two electrons and achieve a noble gas configuration. Sulfur’s electron arrangement—specifically [Ne] 3s² 3p⁴—gives it four unpaired electrons in the 3p subshell, making these valence electrons critical to its chemistry.
At its core, sulfur’s four valence electrons dictate its chemical personality. These electrons are relatively loosely bound compared to inner-shell electrons, allowing sulfur to readily participate in covalent bonding. The presence of four valence electrons enables sulfur to form up to four bonds in molecules, most commonly as part of sulfate (SO₄²⁻), sulfide (S²⁻), sulfite (SO₃²⁻), and sulfur dioxide (SO₂).
This flexibility underpins its role in key compounds—from the formation of life-sustaining amino acids to industrial applications in fertilizers and alloys. “Sulfur’s four valence electrons are the engine behind its dynamic chemistry,” notes Dr. Elena Marquez, a molecular chemist at the National Institute of Standards and Technology.
“They render sulfur both highly reactive and integral to essential biological processes.”
Breakdown of sulfur’s electron configuration reveals why four valence electrons are so significant. Electrons occupy shells and orbitals in a specific order, filling lower-energy levels first. The third shell contains five orbitals, two of which can hold only two electrons each, leaving room for four electrons to reside in the 3p orbitals.
This configuration—S: 3s² 3p⁴—means sulfur has only two electrons missing to fill its third shell completely, a hallmark of Group 16 behavior. This partial completion drives its drive to bond: gaining two electrons is energetically favorable, as it leads toward a stable duet achieved through compound formation.
Beyond basic bonding, sulfur’s four valence electrons influence its oxidation states, which can range from –2 to +6.
This variability stems from enabling sulfur to either gain two electrons (forming sulfide ions or adding to metals) or lose electrons in higher oxidation states—though loss is less common due to the element’s stable core. The +6 state, observed in compounds like sulfur tetrafluoride (SF₄), involves sulfur expanding its valence shell beyond eight electrons through hypervalency, facilitated by d-orbital participation. Yet even here, the initial four valence electrons remain central, guiding electron redistribution during chemical transformations.
The importance of sulfur’s four valence electrons extends into everyday life and global systems. In biology, sulfur is a cornerstone of amino acids such as methionine and cysteine—building blocks of proteins that enable enzyme function, structural support in collagen, and cellular signaling. Without these four valence electrons, sulfur could not form the thiol (-SH) groups essential to enzyme catalysis and redox reactions.
In agriculture, ammonium sulfate fertilizers rely on sulfur’s ability to contribute essential nutrients via its valence electrons, supporting plant growth across continents. Industrially, sulfur’s reactivity—fueled by its four valence electrons—drives applications in vulcanizing rubber, forming sulfuric acid (the world’s most produced industrial chemical), and producing fire retardants and dyes.
Environmental cycles further highlight the significance of sulfur’s valence electrons.
Sulfur moves rapidly through Earth’s systems via oxidation states and redox reactions—processes directly enabled by the availability and transfer of its four valence electrons. Sulfate-reducing bacteria, for example, mediate sulfur transformations in wetlands and ocean sediments, cycling between oxidized (SO₄²⁻) and reduced forms through electron gains and losses, a journey rooted in sulfur’s electronic flexibility. Similarly, volcanic emissions release sulfur dioxide into the atmosphere, where its valence electrons participate in reactions forming acid rain, illustrating how fundamental electron properties shape planetary chemistry.
Understanding sulfur’s four valence electrons is more than academic—it is foundational to unlocking the element’s utility and hazards. The same electrons that make sulfur vital to life also contribute to toxic compounds like hydrogen sulfide (H₂S) when mismanaged. Balancing its beneficent roles with environmental and industrial challenges requires deep insight into this electron configuration.
“Every chemical reaction involving sulfur hinges on those four valence electrons,” asserts Dr. Raj Patel, a geological chemist specializing in sulfur cycling. “Their behavior is predictable yet profound—this is why sulfur remains a linchpin in both natural science and human innovation.” The answer—four valence electrons—belies the complexity behind sulfur’s behavior.
From stabilizing biological macromolecules to driving industrial chemistry, these electrons are not just a number, but the foundation of sulfur’s dynamic, transformative presence in the world. Their role in bonding, electron transfer, and compound formation makes sulfur one of chemistry’s most consequential elements, deserving of precise understanding grounded in both science and real-world application.

Related Post

Unlock Discord’s Full Potential: Master Development with the Official Developer Portal Guide

Winchester, WV Zip Codes Your Complete Guide: Navigating Postal Precision in Hampshire County

Tinola Manok: The Heart of Filipino Family Dining—Authentic Recipe That Delights Every Bite

Unlocking Meaning Stock: How Businesses Are Valuing Purpose Beyond Profit

