Ethanol Chemical Formula
What connects renewable energy, everyday beverages, and cutting-edge industrial chemistry? The answer lies in ethanol — a compound whose chemical formula, C2H5OH, underpins a staggering array of applications from biofuels to pharmaceuticals. Ethanol, the primary alcohol produced through fermentation or catalytic processes, is far more than a simple intoxicant; it is a versatile chemical building block central to modern chemistry and sustainability efforts.
This article explores ethanol’s formula, synthesis, industrial uses, health implications, and emerging innovations, revealing why C2H5OH remains a molecule of immense scientific and practical significance.
Unlocking the Chemistry: The Molecular Structure of Ethanol
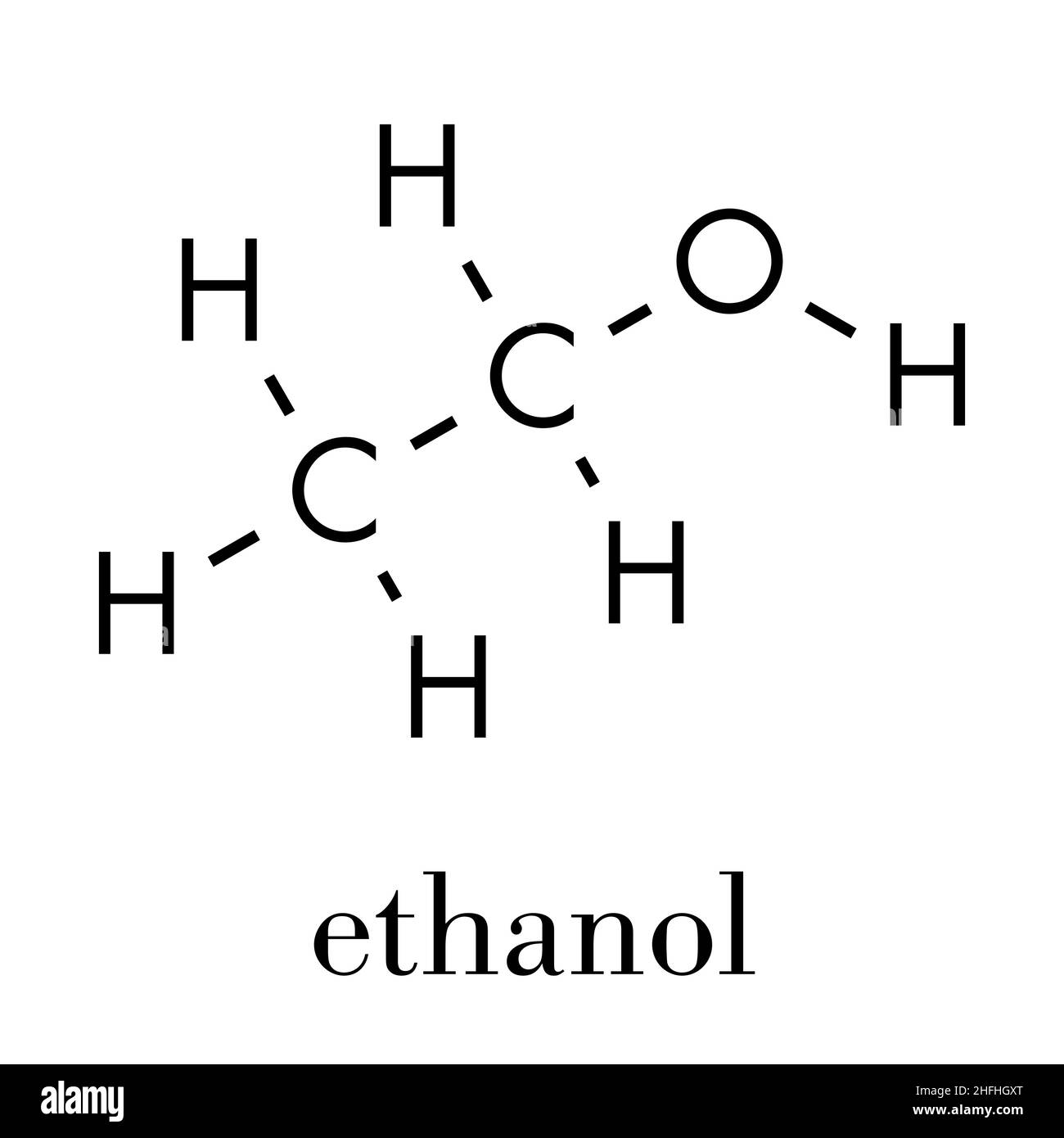
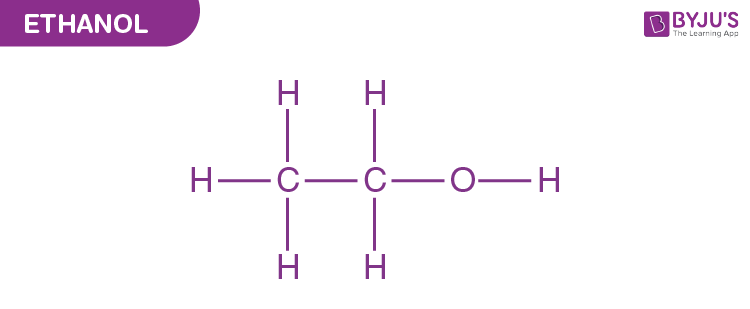
At its core, ethanol is a simple organic compound composed of two carbon atoms, six hydrogen atoms, and one hydroxyl group, symbolized chemically as C2H5OH. This structure defines ethanol’s chemical behavior and reactivity. The molecule features a planar ethyl backbone (–CH2–CH3) with a polar hydroxyl (–OH) group attached to the primary carbon, creating opportunities for hydrogen bonding, solubility in water, and participation in dehydration and esterification reactions.Understanding ethanol’s formula is essential for grasping its role in both nature and industry. The balance between saturated hydrocarbon (ethyl) and polar alcohol functional groups allows ethanol to interact where other hydrocarbons cannot — dissolving in water, engaging in metabolic pathways, and serving as a substrate for enzymatic and catalytic transformations. ${C_2H_5OH}$ is not just a molecular tag; it is a passport to diverse chemical reactivity.
Production Pathways: From Fermentation to Catalysis
Ethanol is most commonly produced through the fermentation of sugars by yeast, a process deeply rooted in biotechnology.In this biological route, microorganisms convert glucose or sucrose into ethanol and carbon dioxide, a reaction summarized as:
C6H12O6 → 2 C2H5OH + 2 CO2
This natural fermentation process powers the global bioethanol industry, supplying renewable transportation fuels and reducing dependence on fossil hydrocarbons. Yet, ethanol’s industrial footprint extends beyond biology. Through catalytic dehydration of ethanol—a process typically employing acidic catalysts under heat—hydrogen is removed to form ethylene, a key petrochemical feedstock.
Further hydrogenation converts ethylene back into ethanol or directly into ethane, illustrating ethanol’s dual role as both product and precursor.
Technological advances in enzymatic catalysis and continuous-flow reactors are improving efficiency and lowering energy demands, making ethanol production increasingly sustainable.
Industrial Applications: Ethanol as a Chemical Workhorse
Ethan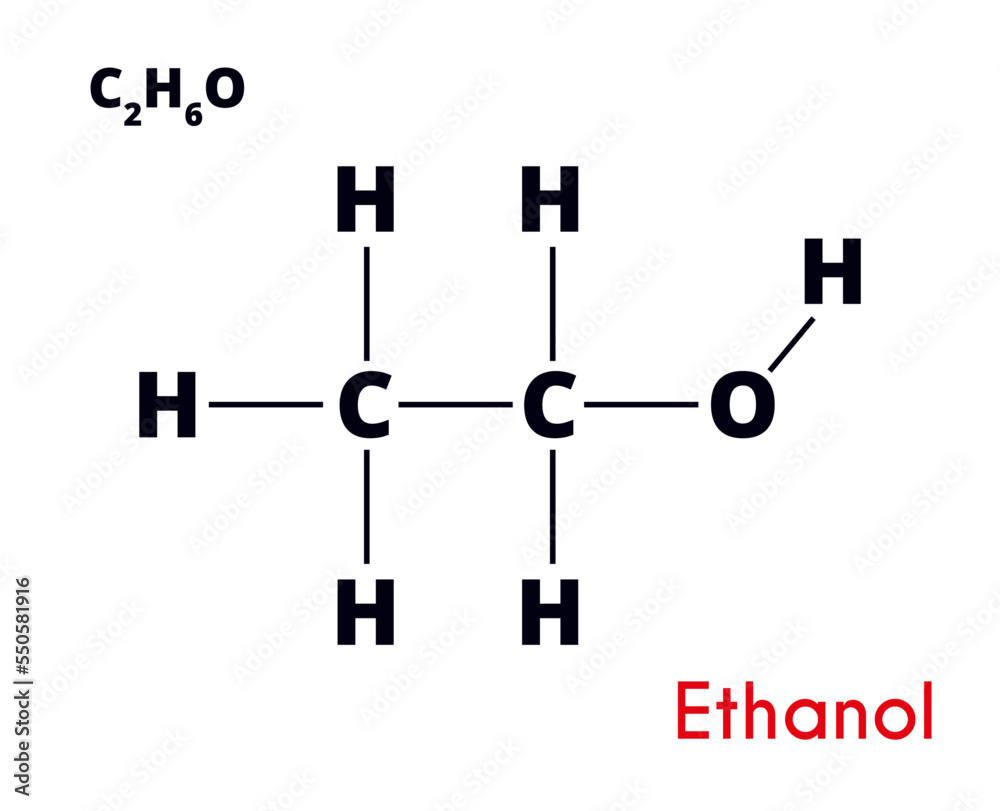

Related Post

The Transformative Power of Trace Letters: Decoding a Tool That Shapes Communication and Learning

From Vince to Vibes: How Dallas vs Packers Memes Reflect NFL Fandom’s Absurd Comedy

Nuel Behi Reveals the Twitter Personality: Decoding the Digital Self Behind the Screen

From Humble Beginnings to Artistic Mastery: The Life and Career of Matthew Patrick

