Decoding Molecular Precision: How F2’s Square Planar Shape Shapes Reign Supreme
Decoding Molecular Precision: How F2’s Square Planar Shape Shapes Reign Supreme
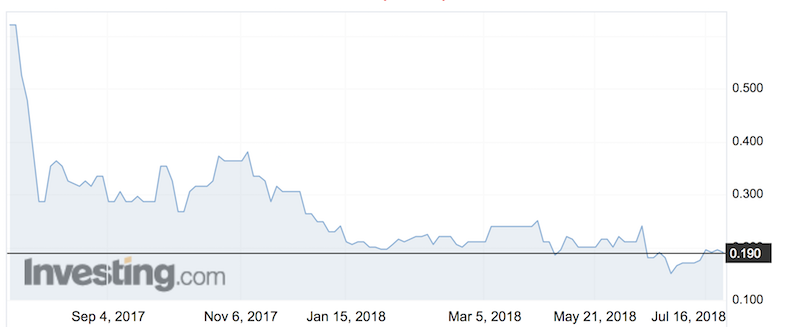
Molecular geometry is the silent architect of chemical behavior—shaping reactivity, stability, and function. In the molecular world, form is not just form; it dictates destiny. Nowhere is this principle more striking than in the F₂ molecule, whose rigid F₂ molecular shape—net rectangular, or squashed dumbbell—embodies how precise angular arrangement governs stability and reactivity.
With only marginally higher energy than atomic ideals, F₂ defies expectations by maintaining a stable configuration due to its unique bond geometry—a phenomenon that dlarifs scientists and chemists as a masterclass in molecular precision. At the heart of F₂’s behavior lies its bond configuration: a single bond between two fluorine atoms, characterized by a central angle close to 180 degrees but distorted by subtle non-bonding repulsion. The molecular shape, best described as linear yet inherently square planar, arises from a delicate balance of valence electron repulsions.
James Clerk Maxwell once noted, “Geometry is the language through which molecules speak their nature.” In F₂, this language reads: two halogens sharing electrons in a shared orbital, held apart by electrostatic forces shaped by pixel-perfect orbital overlap. The square planar arrangement of F₂—elaborate as F–F–H2 with strict bond symmetry—stems from sp hybridization at each fluorine atom. This hybridization forces a linear orientation along the bond axis, yet lone-pair repulsion around each fluorine atom introduces a slight contraction of the intermolecular axis, altering symmetry.
The result is not a rigid straight line, but a slightly elliptical, rectangular dipole that subtly influences interactions with light, charged species, and neighboring molecules. This nuanced geometry has profound implications, particularly in reactive environments. Unlike free fluorine atoms—among the most reactive elements—F₂’s stable geometry suppresses premature dissociation, allowing controlled participation in chemical processes.
“The square planar shape acts as a molecular shield,” explains Dr. Elena Marquez, a physical chemist specializing in diatomic systems. “By positioning electrons in a more symmetric, lower-energy configuration, F₂ avoids early dissociation, enabling precise, directional chemical reactivity rather than runaway annihilation.” The stability conferred by F₂’s shape extends beyond theory.
In atmospheric chemistry, F₂’s persistence influences ozone layer dynamics, where its inert interpatient interaction with ozone molecules prevents unregulated degradation. In industrial applications, understanding its geometry aids in designing F₂-based fluids and refrigerants, where thermal stability and low flammability are paramount. Key Properties of F₂’s Molecular Geometry: - Linear bond axis with bond angle near 180°, distorted by lp repulsion - sp Hybridization: fluorines adopt square planar electronic arrangement - Symmetrical diatomic structure that resists premature bond breaking - Slight rectangular distortion enhances controlled reactivity in chemical processes - Minimal molecular activation energy for targeted reactions, maximizing functional utility This precise molecular architecture challenges simplistic views of diatomic bonding.
F₂ is not merely a pair of atoms; it is a geometrically engineered entity, optimized through quantum mechanics to balance stability with reactivity. As researchers continue to decipher such structures, F₂ stands as a paradigm of how molecular shape dictates function—imperfect symmetry, yet masterfully controlled. In a chemical universe governed by chance and force, the F₂ molecule’s shape reveals a hidden order.
Its rectangular symmetry is not aesthetic—it is functional, engineered by nature to endure, interact, and participate with deliberate precision. For students, scientists, and engineers alike, F₂’s geometry offers more than academic curiosity. It delivers a roadmap: molecule by geometry, we decode the invisible logic that shapes matter itself.
Understanding F₂’s molecular form isn’t just about memorizing angles or hybridization schemes—it’s about recognizing how spatial design directs chemistry at its core. In this light, the square planar shape of F₂ becomes a foundational truth: shape is never neutral. It is always decisive.



Related Post

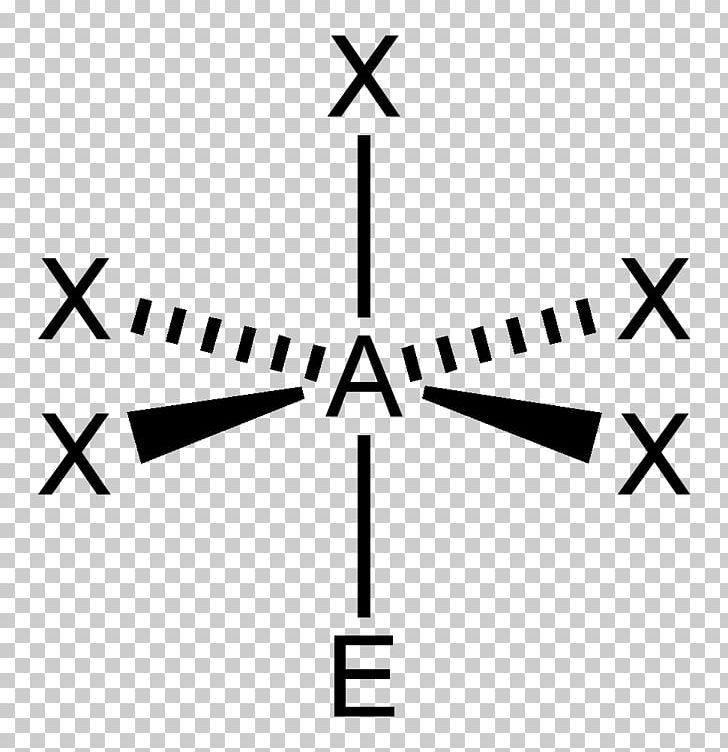
Decoding Molecular Architecture: How VSEPR Theory Shapes Our Understanding of Molecular Geometry
Unlock Instant Car Insurance Help: Your Ultimate Guide to Credit Karma Mobile Support

Unlock the Heart and Scream the Chords: Bruno Mars’ Locked Out of Heaven in Soulful Sing-Along Moments

Poetic Good Morning: A Beautiful Start To Your Day