Decoding Fire: The Precise Science of Combustion Reactions
Decoding Fire: The Precise Science of Combustion Reactions
At the heart of every flame lies a fundamental chemical truth: combustion is a rapid exothermic reaction between a fuel and an oxidizer—most commonly oxygen—expressed mathematically through the EquationForCombustionReaction. This equation, though deceptively simple in form, underpins everything from engine efficiency to wildfire behavior. Understanding its components, stoichiometry, and real-world applications reveals the intricate choreography of energy release that powers modern society and fuels natural phenomena alike.
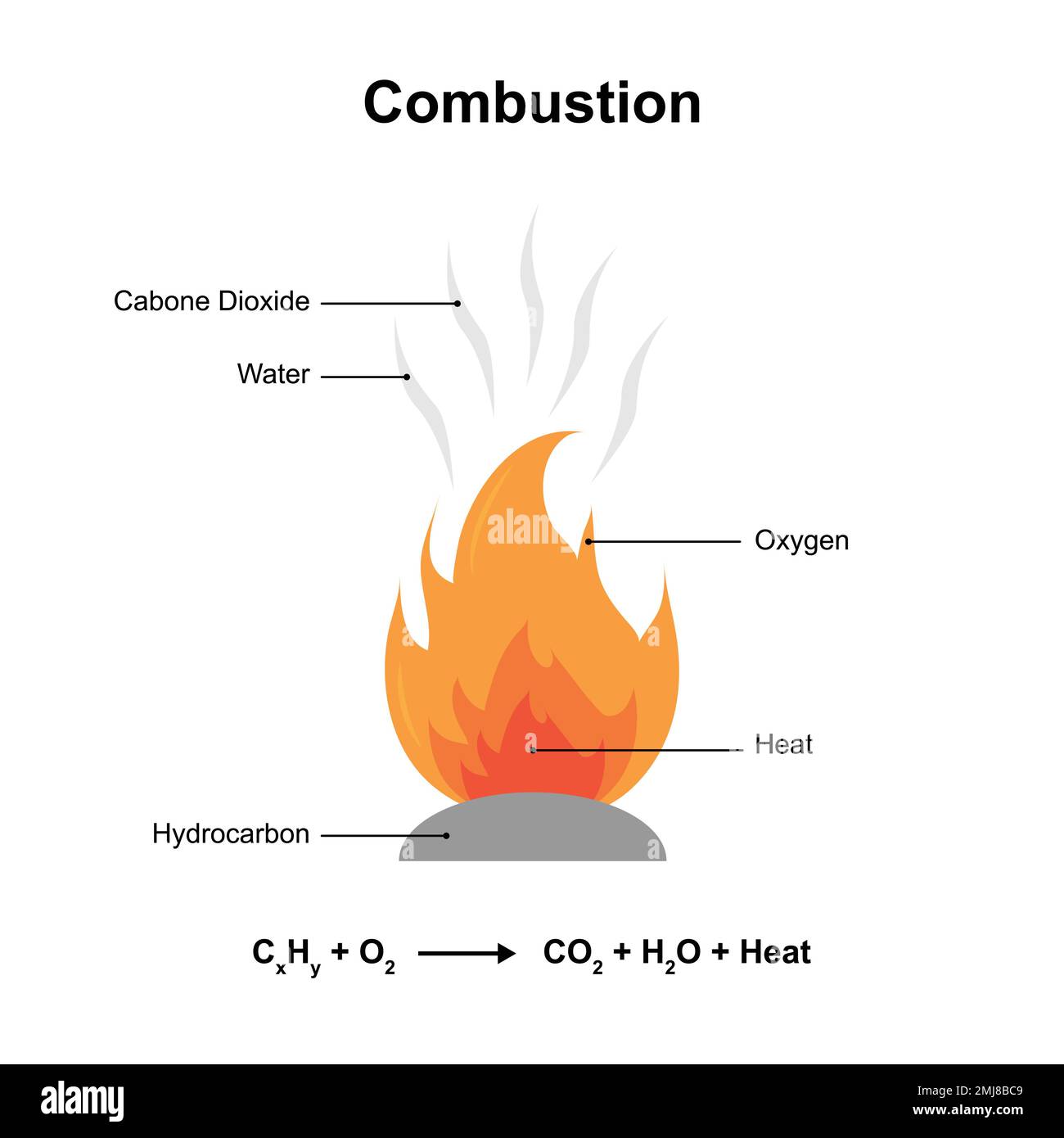
The core of combustion lies in the molecular transformation captured by the standard combustion equation: EquationForCombustionReaction = Fuel + O₂ → CO₂ + H₂O + Energy (often simplified as: Hydrocarbons + O₂ → CO₂ + H₂O + Heat) In balanced form, this reaction reflects conservation of mass and energy, with carbon and hydrogen in hydrocarbon fuels reacting with oxygen to form carbon dioxide and water vapor, releasing vast quantities of thermal energy.“Combustion is nature’s most efficient heat engine,” notes Dr. Elena Vargas, a chemical engineer specializing in energy systems. “Each molecule’s stored chemical energy is unlocked through precise atomic rearrangements governed by well-defined stoichiometric laws.” Understanding this equation requires unpacking its key variables.
The left side defines reactants: a fuel molecule—such as methane (CH₄), octane (C₈H₁₈), or propane (C₃H₈)—paired with diatomic oxygen (O₂), delivered in optimal molar ratios. The right side lists products: carbon dioxide (CO₂), water vapor (H₂O), and—critically—heat energy released. This energy, quantified through enthalpy change (ΔH), determines the reaction’s exothermic nature, making combustion a primary source of power across transport, industry, and power generation.
Balancing the Reaction: Stoichiometry That Drives Efficiency
Stoichiometry—the quantitative relationship between reactants and products—is central to controlling combustion efficiency and emissions. A balanced equation ensures no atom is wasted or overproduced, enabling precise fuel-air mixing. For methane, the cleanest hydrocarbon, the ideal ratio is:Deviations from stoichiometric ratios lead to incomplete combustion, forming harmful byproducts like carbon monoxide (CO) or soot. > “Using real-time sensor data, engineers adjust fuel injection and air flow to maintain stoichiometric flamo-thermodynamic equilibrium,” explains Dr. Marcus Lin, a combustion analyst at the National Energy Lab.
“When the air-fuel mixture is balanced, maximum energy output is achieved with minimal pollutant formation.” Real-world applications depend heavily on this balance. Internal combustion engines, for instance, operate near stoichiometric conditions during ideal combustion to optimize fuel economy. Conversely, industrial furnaces often run lean—excess oxygen—to reduce emissions and prevent overheating.
Thus, EquationForCombustionReaction isn’t just an academic formula; it’s a dynamic tool for engineering precision.
From Vehicles to Wildfires: Diverse Applications of Combustion Equations
The power of the combustion reaction manifests across scales—from microscopic fuel cells to sprawling forest fires. In gasoline engines, hydrocarbon fuels combust in cylinder chambers, converting chemical energy to mechanical motion.The stoichiometric equation guides engineers in tuning ignition timing and air intake, maximizing power while minimizing unburnt residues. In aviation and ship propulsion, high-efficiency combustion chamber designs rely on finely controlled fuel-air ratios derived from EquationForCombustionReaction. Meanwhile, in power plants, coal and natural gas combustion—often partially balanced for heat output—drives turbines to generate electricity.
Yet combustion is not confined to engineered systems. Wildfire behavior follows similar principles. A wildfire’s rapid spread depends on available fuel load, ambient oxygen, and fuel moisture.
“Environmental conditions shift the effective stoichiometry of forest fires,” states fire chemist Dr. Naomi Torres. “Dry fuel and abundant oxygen accelerate combustion, resulting in intense heat pulses that drive fire fronts.”
The Necessity of Precision Combustion reactions demand strict adherence to stoichiometric and environmental conditions. Even small imbalances disrupt energy output and increase emissions. Modern technologies integrate real-time sensors and computational fluid dynamics to monitor and adjust combustion in real time.
This precision is crucial as global fuel use evolves—shifting toward biofuels, hydrogen blends, and synthetic derivatives—each with distinct combustion characteristics. “Hydrogen combustion, for example, burns faster with no CO₂—but requires rethinking ignition and air supply dynamics,” warns Dr. Vargas.
“The EquationForCombustionReaction remains our guide, but its application demands continuous adaptation.” In essence, combustion is the invisible engine of modern activity—powered by a deceptively simple equation that governs energy release at every scale. From lab-scale fuel cells to planetary wildfire outbreaks, understanding and controlling this reaction shapes efficiency, safety, and sustainability. As science advances, so too does our ability to harness combustion—responsibly, efficiently, and cleanly.
/methanecombustion-58e3e6005f9b58ef7e0daa10.jpg)


Related Post
Wichita Zip Code 67201: The Rise, Rhythms, and Rural-Urban Fusion of a Kauffman-Story District

Mastering Letter S Sample 4 Session 6: The Key to Unlocking ELS Reference Skills

The Hidden Power Behind Wordle’s Word Choices: Decoding the Science of Letters, Patterns, and Cognitive Tricks
Aurora Store’s Guide to Open Source App Alternatives: Build Freedom into Your Digital Life

