Decoding Ethane’s Structure: How Lewis Structure for Ethane Reveals Molecular Secrets
Decoding Ethane’s Structure: How Lewis Structure for Ethane Reveals Molecular Secrets
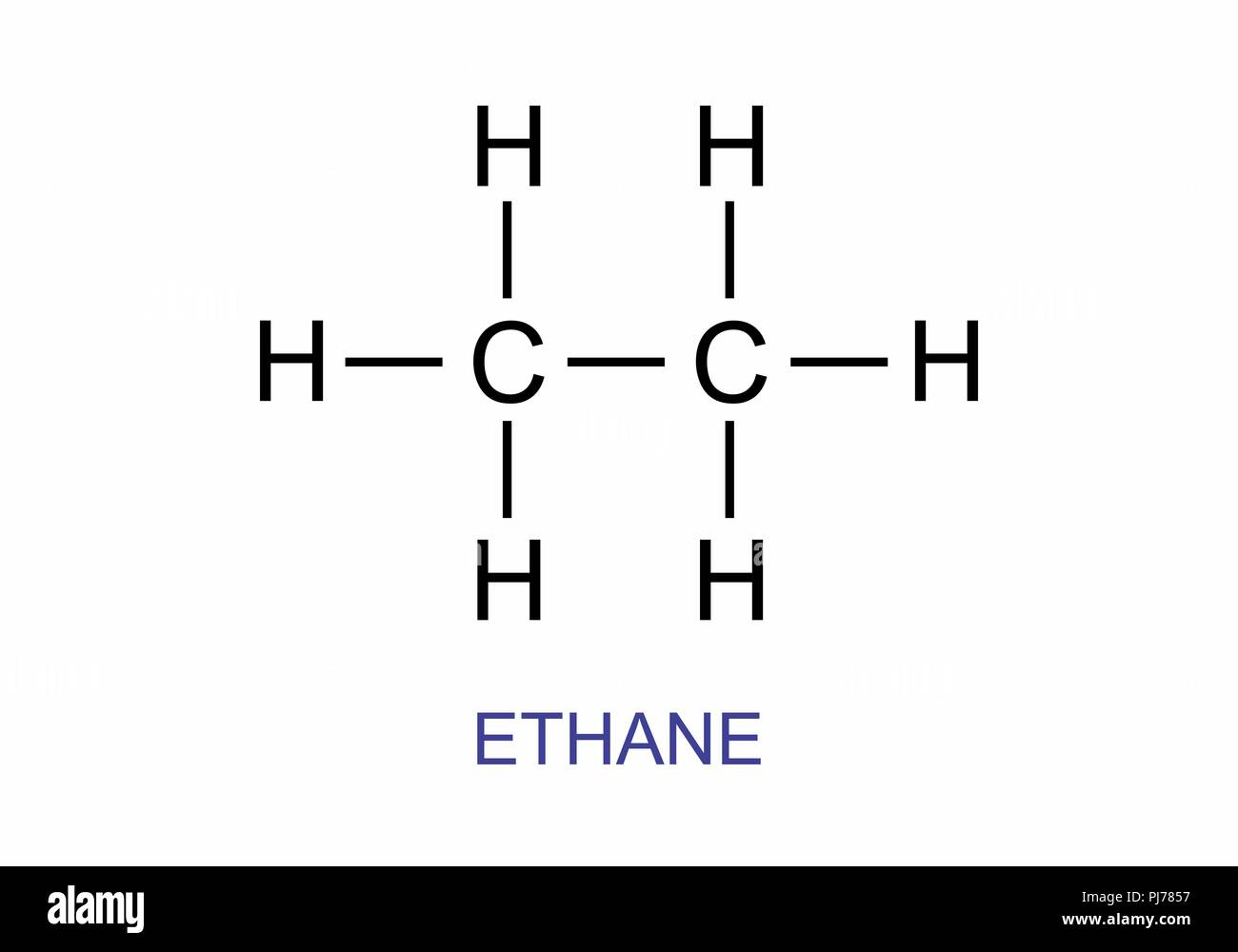
Ethane (C₂H₆) may seem a simple hydrocarbon at first glance, yet its molecular architecture, revealed through the refined principles of Lewis dot symbolism, unfolds a surprisingly intricate world of bonding and stability. Leveraging tools like LewisDotForEthane allows chemists and students alike to visualize the electron arrangement that underpins ethane’s chemical behavior—from its saturated C–C single bond to its legacy in energy and material science. Understanding ethane’s Lewis structure is not just academic—it’s foundational to grasping alkane reactivity, combustion efficiency, and synthetic applications across industries.
At its core, ethane consists of two carbon atoms covalently linked, each bonded to three hydrogen atoms.
The application of Lewis dot theory enables precise representation of valence electrons, forming a clear map of bonding and lone pairs. Carbon, with four valence electrons, forms four single bonds, while hydrogen—each with one electron—shares one electron per bond. The result?
A stable molecule defined by a central C–C σ bond and six shared electrons in three discrete bonds. “This simple pairing illustrates how localized electron sharing governs molecular integrity,” notes a principle embedded in Lewis notation. The structure avoids formal charges across atoms, affirming ethane’s thermodynamic stability and molecular inertness under standard conditions.
The Chemical Foundation of Ethane’s Stability
Lewis dot diagrams for ethane clearly depict the sp³ hybridization of each carbon atom—a key determinant of its tetrahedral geometry and spatial orientation.
With four regions of electron density, carbon distributes its orbitals to minimize repulsion, resulting in optimal bond angles close to 109.5°. This hybridization directly influences physical properties: ethane remains a liquid at room temperature, dense, and with low polarity, properties deeply rooted in its electron-pair distribution. “The symmetry and strain-free arrangement seen in ethane’s Lewis structure explain why alkanes are so widely studied,” explains a structural chemist, “they represent nature’s simplest, most reliable carbon complexes.”
Visualizing Bonds and Reactivity Through Lewis Notation
Beyond formal stability, the Lewis structure for ethane illuminates potential reactive scenarios.
Though the C–C bond is a single σ-bond with low dissociation energy, the notation aids predicting weak points—such as when external energy promotes bond cleavage during combustion. Ethane’s three C–H bonds, each with a shared electron pair, suggest susceptibility to oxidation, a process central to hydrocarbon fuel utilization. Comparing ethane’s structure to unsaturated hydrocarbons like alkenes highlights how saturation yields greater chemical robustness—a critical insight for energy and polymer chemistry.
“Lewis dots do more than depict electrons; they disclose reactivity pathways,” says Dr. Elena Marquez, materials scientist, “showing where energy input transforms inert molecules into active species.”
Applications Driven by Molecular Clarity
The insights from LewisDotForEthane extend beyond theory into industrial practice. In petrochemical refining, understanding ethane’s stable structure guides catalytic cracking processes that convert larger hydrocarbons into valuable ethylene—ethane’s reactive cousin.
Ethylene’s formation from saturated ethane hinges on breaking C–C bonds, a process visually guided by Lewis structure analysis. Similarly, in environmental science, the molecule’s low reactivity contrasts with ozone-depleting chlorofluorocarbons, underscoring why ethane resists degradation in air. “Accurate Lewis representations enable precise modeling of reaction dynamics, emissions behavior, and lifecycle analysis,” argues a green chemist, “helping engineers design cleaner, more efficient fuel systems.”
Lewis Structure as an Educational Gateway
To students and researchers alike, ethane’s Lewis diagram serves as a foundational bridge from abstract electron concepts to tangible molecular reality.
By tracing each bond from shared electron pairs to directional orbitals, learners internalize bonding patterns that underpin organic chemistry, physical property prediction, and thermodynamic modeling. The clarity of Lewis notation demystifies electron flow, making it easier to grasp topics like polarity, hybridization, and molecular orbital theory that follow. “Mastering ethane’s structure isn’t just about memorizing formulas—it’s about seeing the invisible framework that shapes chemical behavior,” observes a chemistry educator.
“That visualization power is what makes Lewis dots indispensable.”
The Lewis dot structure for ethane, though deceptively simple, is a linchpin in understanding carbon-based chemistry. From its precise electron accounting and sp³ hybridization to its role in fuel science and environmental studies, this molecular blueprint reveals how minimal structural elements govern macroscopic properties. Tools like LewisDotForEthane empower learners and professionals to decode ethane’s essence—transforming a basic hydrocarbon into a gateway for exploring the broader chemistry of life’s fundamental building blocks.
As science advances, the clarity and utility of this approach remain unmatched, proving that even basic structural representations hold profound significance in the molecular age.



Related Post

Charles Hill Global Business Today: Navigating the New Frontiers of International Commerce
Transform Your Content Instantly with Ispoofer.App: The Ultimate AI-Powered Editing Tool

The Most Hated Zodiac Sign: Unveiling the Truth Behind Astrological Dislikes

Jigsaw Explorer: Revolutionizing Digital Research One Click at a Time

